Table of Contents


Table of Contents
For Richard, Sam and Matt

This edition first published 2013 © 2013 by Sue Paterson and Karen Tobias
Wiley-Blackwell is an imprint of John Wiley & Sons, formed by the merger of Wiley’s global Scientific, Technical and Medical business with Blackwell Publishing.
Registered office: John Wiley & Sons, Ltd, The Atrium, Southern Gate, Chichester, West Sussex, PO19 8SQ, UK
Editorial offices: 9600 Garsington Road, Oxford, OX4 2DQ, UK
The Atrium, Southern Gate, Chichester, West Sussex, PO19 8SQ, UK
2121 State Avenue, Ames, Iowa 50014-8300, USA
For details of our global editorial offices, for customer services and for information about how to apply for permission to reuse the copyright material in this book please see our website at .
The right of the author to be identified as the author of this work has been asserted in accordance with the UK Copyright, Designs and Patents Act 1988.
All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, except as permitted by the UK Copyright, Designs and Patents Act 1988, without the prior permission of the publisher.
Designations used by companies to distinguish their products are often claimed as trademarks. All brand names and product names used in this book are trade names, service marks, trademarks or registered trademarks of their respective owners. The publisher is not associated with any product or vendor mentioned in this book. This publication is designed to provide accurate and authoritative information in regard to the subject matter covered. It is sold on the understanding that the publisher is not engaged in rendering professional services. If professional advice or other expert assistance is required, the services of a competent professional should be sought.
Library of Congress Cataloging-in-Publication Data
Paterson, Sue.
Atlas of ear diseases of the dog and cat / Sue Paterson & Karen Tobias.
p. cm.
Includes bibliographical references and index.
ISBN 978-1-4051-9326-9 (hardcover : alk. paper) 1. Dogs–Diseases–Atlases. 2. Cats–Diseases–Atlases. 3. Ear–Diseases–Atlases. I. Tobias, Karen M. II. Title.
SF991.P375 2013
636.089'78–dc23
2012010185
A catalogue record for this book is available from the British Library.
Wiley also publishes its books in a variety of electronic formats. Some content that appears in print may not be available in electronic books.
Figures 1.15, 1.16, 1.24, 1.37, 1.38, 1.39b, 1.41, 7.1 and 7.23 were illustrated by Samantha Elmhurst.
Cover design by hisandhersdesigns.
FOREWORD
My interest in ear diseases began during my first job as a new veterinary graduate in Florida, in 1978. It seemed as if almost every other dog that I saw had ear disease. In those days, otic medications were limited and ear problems rarely came under control easily.
Over the last 34 years, my interest in trying to understand ear disease has led me to write 2 ear disease books, lecture at over 350 veterinary conferences worldwide, help develop diagnostic and therapeutic tools, and teach veterinarians to use video otoscopy in their practices. I have had the honor of lecturing with Sue Paterson and in my opinion, she has an amazing way of captivating an audience with such an obscure topic as ear diseases.
The evolution of improved otic diagnostics allows us to actually see and work in the ear canal much easier than in the past. We are now able to identify tumors and middle ear disease, which are quite prevalent in both dogs and cats. New treatments of ear diseases in the dog and cat based on scientific evidence help our patients recover faster. Our pet owning clients are grateful to us for the comfort of their pets.
Through a collaborative effort, many veterinarians both in academia and in private practice have chosen to pursue their interest in veterinary otology bringing new ideas, exciting research, new drugs, and new surgical techniques, such as laser surgery, to the practice of otology. In their important contribution to the veterinary literature, Sue Paterson and Karen Tobias share with us a detailed atlas based upon their clinical experiences to help us understand the anatomy, physiology, and pathogenesis of ear disease. Sue Paterson also shares with us her unique knowledge as well as her experience in the area of hearing and audiology in the dog.
As our profession changes, we should strive to improve our diagnostic skills to survive in the competitive environment that exists presently. Learning about ear disease, acquiring the knowledge and the skill necessary to do good ear work serves to increase the value of you personally as an animal healer. Proper ear disease treatment will solidify your clients to your veterinary practice. The value of otology cannot be overemphasized in today’s small animal practice.
Louis N. Gotthelf, DVM
Montgomery, Alabama USA
PREFACE
It has been suggested that ear cases may make up more than 10% of the typical companion animal veterinarian’s case load, so the ability to manage ear disease well is essential for the primary care veterinarian. Otology is one of the most rapidly expanding branches of small animal medicine and surgery and although there are many excellent textbooks already available on this subject, this is the first – and I hope one of the most complete – illustrated atlases of ear disease. I am grateful that Wiley-Blackwell has indulged my fascination for ear disease by allowing me to compile it. Ear disease for me is a passion: not only is it the challenge to diagnose and treat the dogs and cats that present to me every day in the clinic but also the challenge to pass on knowledge to fellow professionals, so that the care of ear disease in the species we deal with can be improved. The pain that otitis can cause is in my opinion often underestimated. Frequently it is only after medical therapy has resolved disease, or radical surgery has removed an ear canal, that owners can recognise how much discomfort their pet has been in, as they perceive dramatic changes in their dog’s or cat’s behaviour. Ear disease should never go untreated; even with the financial constraints placed on us by many of our clients we have a duty to make animals with otitis comfortable.
Working with Karen Tobias, a true goddess of surgery, has been a joy and an honour and to be able to include her enormous wealth of surgical experience in this book has enhanced it hugely. I recognize that she has spent hours preparing pictures and text that will hopefully help guide inexperienced clinicians through basic techniques and provide detailed explanation for the more experienced surgeons of the more complex procedures. I know she has enjoyed preparing her sections of the book as much as I have mine. Thanks, Karen.
Sue Paterson
ACKNOWLEDGEMENTS
There are numerous people who have helped and guided me throughout my dermatological career; however, the person who must take the most responsibility for kindling my interest in ear disease is Craig Griffin, a true master of his art and an inspirational teacher. Thanks must go to my two business partners, Ian and Duncan who as surgeons have indulged my dermatological madness for years. I hope all the dermatology nurses at work, strictly alphabetically Bernie, Charlotte, Emma and Lydia, know how much I appreciate them; without their hard work my life would be impossible. And of course Janie my practice manager and friend, she knows I can’t manage without her. Finally a huge thanks to all the loyal referring veterinary surgeons who provide me with one of the most rewarding day jobs you could ask for and of course all the material for the book.
Sue Paterson
Doing a total ear canal ablation and bulla osteotomy in a greasy Cocker Spaniel is a lot like cleaning the bathroom – the procedure is not always pleasurable, but the product is much more tolerable. Writing can be like that as well – the impetus to start the process is not always present, and sometimes the distractions prove stronger than the will to persist with the task at hand. In the end, though, the promise of a useful, attractive and educational text provides the drive needed to get the job done. I am grateful to Sue Paterson for providing that promise – who else but a dermatologist would be so excited about greasy Cocker Spaniels? I would also like to acknowledge my administrators and co-workers at University of Tennessee College of Veterinary Medicine for supporting me in this endeavour. Special thanks to photographers Phil Snow and Greg Hirshoren for their contributions and Deb Haines for her contract work and finalization of the images. And, as always, a big thanks to my two talented and attractive offspring, Jacob and Jessica, for their love and encouragement. Ears to you!
Karen Tobias
CHAPTER 1
ANATOMY OF THE EAR
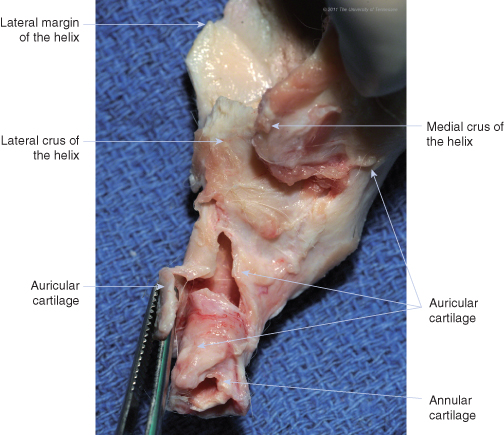
The pinna is the most prominent portion of the external ear (). It has an inner, concave surface and an outer, convex surface. In the standing ear, the concave surface forms a conchal cavity that is directed rostrally or laterally, while the convex surface faces medially or caudally. The distal tip of the pinna is called the apex, and the lateral and medial free margins of the pinna are called the helix (). The rostrolateral boundary of the distal portion of the ear canal is called the tragus. A notch caudal to the tragus, the intertragic incisure, separates it from the antitragus, which is a thin elongated piece of cartilage that extends up to the lateral margin of the helix at the cutaneous marginal pouch.
General anatomy of the pinna. The conchal cavity of the concave surface of the ear can be directed rostrally or laterally.
(Photo by Phil Snow, UTCVM) © 2012 The University of Tennessee.

Concave surface of the right dog pinna. The antihelix and tragus form the boundaries of the ear canal opening.
(Photo by Phil Snow, UTCVM) © 2012 The University of Tennessee.

The margins of the pinna are divided into medial, or rostral, and lateral, or caudal (see ). These variations in directional description can make the anatomy very confusing.
The external ear is composed of three cartilages: annular, auricular, and scutiform. The ear canal is formed proximally (near the skull) by the annular cartilage and distally (away from the skull) by the auricular cartilage, which fans out to form the pinna ().
Auricular and annular cartilage of the right ear of a dog, lateral view.
(Photo by Phil Snow, UTCVM) © 2012 The University of Tennessee.

The auricular cartilage is divided into three sections: the scapha, the concha, and the tubus auris, or conchal tube (). Whereas the scapha is distally located and flattened, the concha is rolled into a trumpet shape to form the conchal cavity (). The scapha and concha are divided on the concave surface by the antihelix, a transverse cartilaginous fold.
Auricular and annular cartilage of the right ear, caudal view. The annular cartilage is nestled within the auricular cartilage, which forms the pinna and vertical ear canal. Note that the proximal portion of the auricular cartilage spirals inward as it bends.
(Photo by Phil Snow, UTCVM) © 2012 The University of Tennessee.

Medial view of cartilage of the right ear. A portion of the auricular cartilage that forms the conchal tube has been elevated; underneath is another extension of auricular cartilage that wraps around the annular cartilage. Note that the ear canal is not a solid funnel: the auricular and annular tubes are each formed by overlapping flaps of cartilage that allow flexibility. Animals with severe otitis externa or conchal obstruction may develop periauricular abscesses from disruption of the fibrous connective tissue sheath surrounding either the tube flaps or the auricular-annular or annular-osseous junction.
(Photo by Phil Snow, UTCVM) © 2012 The University of Tennessee.
The concha forms a funnel shape that thickens proximally as it becomes the conchal tube. The conchal tube forms the vertical ear canal. This canal is up to an inch (2.5 cm) deep and, as it progresses proximally towards the head, is directed ventrally, medially, and slightly rostral, spiralling inwards. It is partially surrounded along its proximal lateral border by the parotid salivary gland.
The annular cartilage is a separate, rolled, cartilaginous band that fits inside of the base of the conchal tube. It forms the horizontal ear canal, which runs medially toward the skull. In turn, the annular cartilage overlaps the osseous external acoustic meatus. Junctions of the auricular and annular cartilages and the annular cartilage and skull are connected by a fibrous tissue sheath. Because of these moveable joints, the auditory canal can be straightened during otoscopic examination. Epithelium lining the auricular and annular cartilage contains sebaceous and ceruminous glands and hair follicles ().
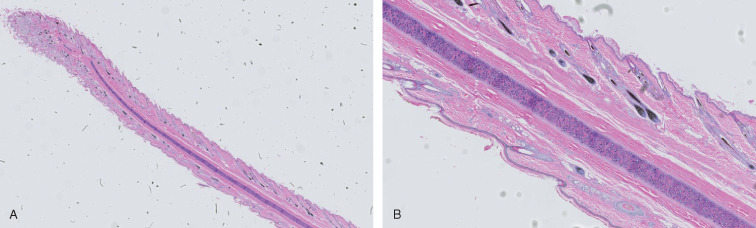
(A), Cross-section of the pinna of a dog. (B), Components including hyaline cartilage, muscle, and hair follicles are easily visible.
(Courtesy, UTCVM Virtual Microscope) © 2012 The University of Tennessee.
Terminology for the ear canal varies within and amongst texts. Some authors consider the osseous extension of the skull that encompasses the tympanic membrane to be the external acoustic meatus or osseous external acoustic meatus, while others consider the external acoustic meatus to be the opening of the conchal tube at the level of the tragus and antihelix. The cartilaginous tube that extends from the meatus to the concha, which is a combination of conchal tube (auricular) and annular cartilage, is sometimes called the auditory canal.
A variety of muscles attach the ear rostrally, ventrally, or caudally to the head (); these muscles are innervated by the facial nerve. Some of these muscles are continuous with the cervical portion of the platysma. The plate-like, L-shaped scutiform cartilage, which is medial to the auricular cartilage, lies within the muscles that attach the auricular cartilage to the head (). By acting as a fulcrum, the scutiform cartilage improves mobility of the auricular cartilage.
Muscles of the canine ear and face: right lateral view.
(Photo by Phil Snow, UTCVM) © 2012 The University of Tennessee.

Muscles of the canine ear and head: dorsal view. The scutiform cartilage is enveloped within the dorsal group of muscles.
(Photo by Phil Snow, UTCVM) © 2012 The University of Tennessee.

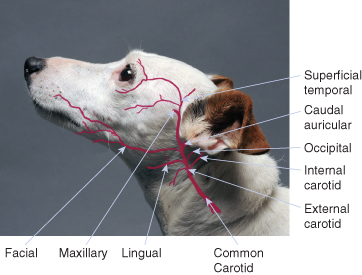
The major portion of the blood supply to the external ear comes from the caudal auricular artery, which arises from the external carotid artery at the base of the annular cartilage and medial to the parotid salivary gland (). The caudal auricular and superficial temporal veins, which terminate at the maxillary vein, provide drainage of the external ear (). Perforations in the auricular cartilage permit passage of blood vessels and nerves from the convex to the concave surface.
Selected branches of the common carotid artery. The external carotid artery gives off the caudal auricular artery and then travels around the ventral and rostral aspects of the horizontal canal before terminating in the maxillary and superficial temporal arterial branches.
(Background photo by Phil Snow, UTCVM) © 2012 The University of Tennessee.
Selected tributaries of the external jugular vein. The superficial temporal vein travels ventrally around the rostral aspect of the horizontal canal and then joins the maxillary vein, which lies ventral to the canal.
(Background photo by Phil Snow, UTCVM) © 2012 The University of Tennessee.

Sensory innervation to the concave surface of the pinna is provided primarily by branches of the facial nerve () and, at the rostral extent of the pinna, by branches of the trigeminal nerve. The lateral auricular branch of the facial nerve provides sensation to the majority of the vertical canal, along with a portion of the horizontal canal, while the auriculotemporal branch of the trigeminal nerve provides sensory innervation to the horizontal canal and tympanic membrane. The convex surface of the pinna receives sensory innervation via the second cervical nerve. Communications between vagal and facial nerve branches may also be present.
Selected branches of the facial nerve. After exiting the stylomastoid foramen, the facial nerve travels near the caudal, ventral, and rostral aspects of the horizontal ear canal.
(Background photo by Phil Snow, UTCVM) © 2012 The University of Tennessee.

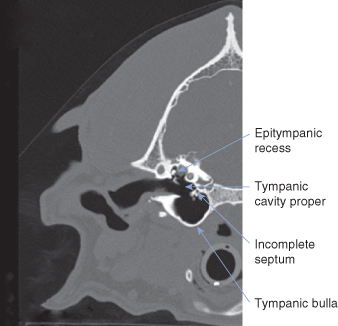
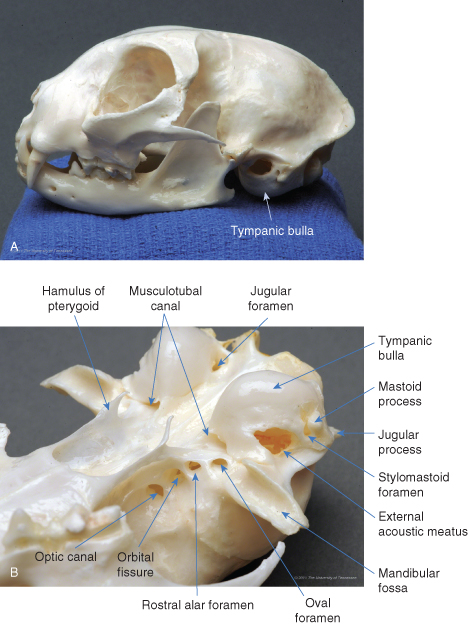
The canine middle ear ( and ) consists primarily of an air-filled tympanic cavity that is separated from the external ear by the tympanic membrane and from the inner ear by the vestibular and cochlear windows. The middle ear is divided into three parts: (1) a large, ventral tympanic bulla within the temporal bone; (2) a small, dorsal epitympanic recess, which sits above the level of the tympanic membrane; and (3) the tympanic cavity proper, which connects the two and is bounded on its lateral surface by the tympanic membrane ().The tympanic cavity proper is partially separated from the ventral tympanic bulla by an incomplete septum. The tympanic cavity proper contains the cochlear (round) window along its caudal aspect. The ossicles of the ear – the stapes, incus, and portions of the malleus – reside within the epitympanic recess and span the distance from the inner ear to the tympanic membrane (). The tympanic cavity is lined by simple squamous or cuboidal epithelium, except at the orifice of the auditory tube.
Left lateral view of the canine skull. In this image, the mandible has been removed and the skull has been rotated slightly. Note how the bulla is less prominent than the retroarticular and jugular processes.
(Photo by Phil Snow, UTCVM) © 2012 The University of Tennessee.

Right lateral view of the canine bulla with the mandible in place. During ventral bulla osteotomy, the position of the canine bulla is estimated by palpating the jugular and angular processes.
(Photo by Phil Snow, UTCVM) © 2012 The University of Tennessee.

Parts of the canine middle ear. In this dog, the manubrium of the malleus is visible as an L-shaped structure, and a portion of the septum can be seen along the medial wall of the bulla.
(Courtesy, UTCVM Radiology) © 2012 The University of Tennessee.
The auditory ossicles – the malleus, incus, and stapes – span the distance from the tympanic membrane to the oval window membrane.

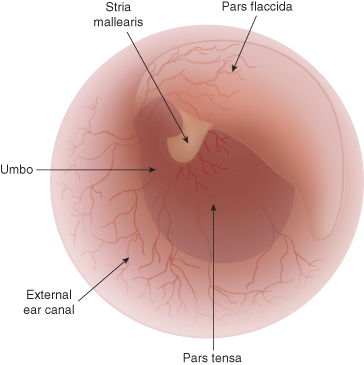
The tympanic membrane is oval in shape and concave from an external viewpoint because of medial traction by the attached malleus (). In the dog it lies at a 45° angle to the long axis of the horizontal canal, with its ventral aspect farther from view than the dorsal portion. The largest portion of the tympanic membrane is called the pars tensa, a taut, semi-transparent, fibrous membrane. The pars tensa is firmly attached to the surrounding osseous external acoustic meatus by the annulus fibrocartilaginous, a fibrocartilage ring. The much smaller, dorsal portion of the tympanic membrane, known as the pars flaccida, is loose, opaque, and richly vascularized ().
Diagram of the tympanic membrane. The tympanic membrane curves away from the external canal because of inward tension of the malleus, which attaches to it. Epithelium regenerates from the umbo outwards; this area should be avoided during myringotomy.
In normal dogs, the pars flaccida may be very prominent and therefore easily confused with a mass.
(Courtesy, UTCVM Dermatology Service) © 2012 The University of Tennessee.

The tympanic membrane is formed by a layer of fibrous tissue covered on its external surface with stratified squamous epithelium and on its inner surface with simple squamous or cuboidal epithelium. The manubrium of the malleus is embedded in the fibrous layer of the membrane (see ), resulting in an inward depression called the umbo. The tympanic membrane regenerates radially from the umbo pars tensa and becomes thicker toward its periphery. Visibility of the malleus through the pars tensa results in a white streak known as the stria mallearis.
The incus and the head of the malleus almost entirely fill the small epitympanic recess (). The malleus has three attachments: the tympanic membrane, petrous temporal bone, and incus. The incus is suspended between the stapes and malleus, and the footplate of the stapes is attached to the membrane over the oval window. The malleus is controlled by the tensor tympani muscle, which originates in the tympanic bulla and is innervated by the tensor tympani nerve, a branch of the trigeminal nerve. Contraction of the tensor tympani muscle makes the tympanic membrane more rigid. The stapedius muscle also originates in the tympanic bulla. It inserts on the stapes and is innervated by the stapedial branch of the facial nerve. The stapedius muscle contracts reflexively with loud noise, decreasing movement of the stapes to protect the ear from damage.
Transverse CT image of a dog skull showing the position of the malleus and tympanic membrane.
(Courtesy, UTCVM Radiology) © 2012 The University of Tennessee.

The promontory is a bony eminence on the dorsomedial wall of the tympanic cavity that houses the cochlea (). The promontory lies opposite of the tympanic membrane and medial to the epitympanic recess. The cochlear (round) window is found in the caudolateral portion of the promontory and opens to the perilymph in the scala tympani of the cochlea. The cochlear window is covered with a thin, secondary tympanic membrane that oscillates to dampen vibrations within the cochlear perilymph. The vestibular (oval) window lies on the dorsolateral surface of the promontory and is covered by a thin membrane to which the foot of the stapes is attached. Facing the vestibular window is a slit-like opening into the facial canal, through which the facial nerve travels.
Ventral view of the right caudal canine skull with the tympanic bulla removed. The vestibular, or oval, window is on the dorsolateral surface of the promontory and just rostral to the cochlear (round) window.
(Photo by Phil Snow, UTCVM) © 2012 The University of Tennessee.

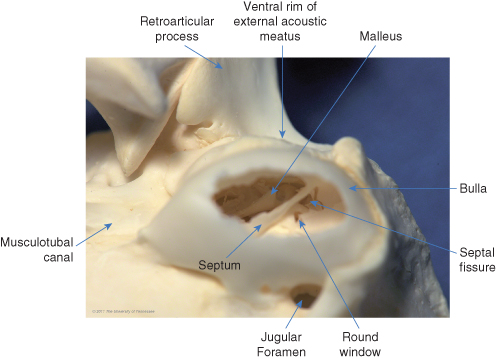
The auditory or eustachian tube connects the tympanic cavity to the nasopharynx (). It is oval in shape, 5–15 mm long, and 1–3 mm in diameter. The auditory tube begins as a short osseous tube – a canal through the temporal bone – that exits the skull rostromedial to the bulla as the musculotubal canal (see ). Within the tympanic cavity, its proximal ostium can be seen on the rostral surface of the tympanic cavity proper. The distal end of the tube is supported by a narrow cartilaginous trough and opens on the lateral wall of the nasopharynx, dorsolateral to the soft palate at its midpoint.
Ventral view of the caudal half of the canine skull (mandible removed). The auditory tube originates from the dorsolateral wall of the bulla (inset), just rostral to the tensor tympani muscle attachment, as the musculotubal canal. The distal end opens rostrally on the dorsolateral wall of the nasopharynx, just medial to the ipsilateral pterygoid process, or hamulus.
(Photos by Phil Snow, UTCVM) © 2012 The University of Tennessee.
The auditory tube functions to equalize pressure across the tympanic membrane. Its distal end can be actively opened through tension by the tensor veli palatini muscle but otherwise remains closed because of surface tension caused by contact between air and mucus. Like the respiratory tract, the auditory tube is lined by ciliated pseudostratisfied columnar epithelium containing goblet cells.
The tympanic cavity is closely associated with several nerves and vessels that can become damaged with middle ear disease or surgical trauma (). The sympathetic postganglionic nerves to the eye and orbit are collectively called the internal carotid nerves. In the dog they travel along with the internal carotid artery through the carotid canal, which is separated from the tympanic cavity by a thin bony plate.
Ventral view of the canine skull (right bulla removed) with diagram of regional nerves. The facial nerve travels through the facial canal, an S-shaped trough in the epitympanic recess, and exits at the stylomastoicd foramen just caudal to the external acoustic meatus. The retroarticular vein, which is the termination of the temporal sinus, exits from the retroarticular foramen, while the internal carotid artery travels under a thin shelf of bone with the internal carotid nerves. The jugular process serves as the caudal attachment site of the digastricus muscle.
(Photos by Phil Snow, UTCVM) © 2012 The University of Tennessee.

The facial nerve travels through the sigmoid-shaped facial canal of the petrous temporal bone. The facial canal is an incomplete tunnel that opens into the tympanic cavity lateral to the vestibular window. The facial nerve leaves the petrous part of the temporal bone through the stylomastoid foramen.
The chorda tympani, a branch of the facial nerve, travels medial to the base of the malleus in the epitympanic recess. It is sometimes called the tympanic nerve. The chorda tympani provides innervation to the mandibular and sublingual salivary glands and fungiform papillae on the rostral two thirds of the tongue. Ipsilateral papilla may atrophy if the nerve on the affected side is damaged from otitis media.
The tympanic plexus lies on the promontory and is formed primarily by fibres from the tympanic branch of the glossopharyngeal nerve (cranial nerve IX). The tympanic branch of the glossopharyngeal nerve supplies innervation to the lining of the tympanic bulla, providing pressure and pain sensation, and to the parotid and zygomatic salivary glands.
The auriculotemporal nerve, a branch of the mandibular nerve, passes medial and caudal to the retroarticular process of the temporal bone and emerges between the base of the auricular cartilage caudally and masseter muscle cranial. One of its branches – the external acoustic meatus nerve – is sensory to the external acoustic meatus near the tympanic membrane. Another branch – the rostral auricular nerve – supplies the skin over the lateral aspect of the tragus and a rostroventral portion of the pinna’s concave surface.
The internal carotid artery () enters the jugular foramen and travels with the sympathetic fibres of the internal carotid nerve via the tympano-occipital or petro-occipital fissure into the middle ear, where it travels through the carotid canal (). It exits the canal at the rostromedial edge of the tympanic bulla at the foramen lacerum. The nearby petro-occipital canal transmits the ventral petrosal venous sinus. Axons of the glossopharyngeal (IX), vagus (X), and accessory (XI) nerves also pass through the jugular foramen and travel in the tympano-occipital fissure.
Relationship of selected arteries with the right bulla of a dog. In this view, a ventral bulla osteotomy has been performed and the manubrium is visible. The internal carotid artery passes through the tympano- (or petro-)occipital fissure, travels within the carotid canal, and passes out and then back into the foramen lacerum before travelling on to the brain.
(Background photo by Phil Snow, UTCVM) © 2012 The University of Tennessee.

Dorsolateral (A) and dorsal (B) views of the open canine skull.
(Photos by Phil Snow, UTCVM) © 2012 The University of Tennessee.

Beyond its bifurcation with the internal carotid artery, the external carotid artery travels rostrally, forming an S shape (see ) that traverses from medial to lateral over the base of the bulla and then rostrally under the external acoustic meatus. It gives off several branches, including the caudal auricular and superficial temporal arteries. The caudal auricular artery circles the caudal half of the ear at the base of the annular cartilage. The superficial temporal artery is found at the rostral extent of the base of the auricular cartilage.
As in the dog, the ossicles in the cat rest high up within the epitympanic recess (). Cats have a much less prominent pars flaccida, so the manubrium is more easily visible through the tympanic membrane (). The bulla is more prominent in cats that in dogs, making it easier to locate by palpation during ventral bulla osteotomy (). In cats, the tympanic cavity is divided into two compartments by a thin bony septum () that runs from a midrostral to a midlateral position (). The dorsolateral compartment is the smaller of the two (). Its lateral wall is comprised primarily by the tympanic membrane, which is oriented perpendicular to the long axis of the horizontal canal. Much of the dorsolateral compartment is occupied by the auditory ossicles immediately medial to the tympanum. The opening of the auditory tube is within the rostromedial aspect of the dorsolateral compartment. The ventromedial compartment, which also extends caudal to the dorsolateral compartment, is primarily an air-filled bulla.
As in the dog, the ossicles of the cat lie within the epitympanic recess and extend from the tympanum to the oval window. A bony septum separates the bulla into two cavities.

The manubrium in this cat is easily visible through the tympanic membrane.
(Courtesy, UTCVM Dermatology) © 2012 The University of Tennessee.

Anatomy of the cat skull. (A) Left lateral view. (B) Right ventrolateral view. The bulla extends ventral to the retroarticular and jugular processes, making it easily palpable.
(Photos by Phil Snow, UTCVM) © 2012 The University of Tennessee.
On the close-up view of the left feline bulla, the septum is visible through the osseous external acoustic meatus.
(Photo by Phil Snow, UTCVM) © 2012 The University of Tennessee.

Ventral view of a cat skull: the floor of the left bulla has been removed (similar to ventral bulla osteotomy) to show the intact septum.
(Photo by Phil Snow, UTCVM) © 2012 The University of Tennessee.

Normal cat CT scan. Note the septum (arrow) that divides the dorsolateral and ventromedial chambers.
(Courtesy, UTCVM Radiology) © 2012 The University of Tennessee.

The septum dividing the bulla is incomplete dorsally: a narrow fissure on the caudomedial aspect of the dorsolateral compartment permits communication with the ventromedial compartment (). The caudal end of the fissure enlarges into a triangular foramen, which is occupied along its medial wall by the cochlear (round) window, which faces laterally. The promontory on the dorsal wall of the bulla is medial to the cochlear window and extends to both sides of the septum.
Ventral view of a cat skull showing interior of the left bulla after partial removal of the septum. A fissure in the caudodorsal aspect of the septum allows connection of the two compartments. The round window is located along the dorsal aspect of the fissure.
(Photo by Phil Snow, UTCVM) © 2012 The University of Tennessee.
). The origin of the auditory tube is found on the dorsomedial portion of the rostral wall of this compartment.