
Contents
Cover
Other Titles Available in the Chemistry at a Glance series:
Title Page
Copyright
Biography
Abbreviations
Introduction to Second Edition
Further Reading
References
Chapter 1: Heterocyclic Nomenclature
Six-Membered Aromatic Heterocycles
Five-Membered Aromatic Heterocycles
Non-Aromatic Heterocycles
Small-Ring Heterocycles
Chapter 2: Structures of Heteroaromatic Compounds
Structures of Benzene and Naphthalene
Structures of Pyridines and Pyridiniums
Structures of Quinolines and Isoquinolines
Structures of Diazines (Illustrated Using Pyrimidine)
Structures of Pyrroles, Thiophenes and Furans
Structure of Indoles
Structures of Azoles (Illustrated Using Imidazole)
Chapter 3: Common Reaction Types in Heterocyclic Chemistry
Introduction
Acidity and Basicity
Electrophilic Substitution of Aromatic Molecules
Nucleophilic Substitution of Aromatic Molecules
Radical Substitution of Heterocycles
C-Metallated Heterocycles as Nucleophiles
Generation of C-Metallated Heterocycles
Dimethylformamide Dimethyl Acetal (DMFDMA)
Formation and Hydrolysis of Imine/Enamine
Common Synthetic Equivalents of carbonyl Compounds in Ring Synthesis
Cycloaddition Reactions
Chapter 4: Palladium in Heterocyclic Chemistry
Palladium(0)-Catalysed (and Related) Reactions
Addition to Alkenes: the Heck Reaction
Carbonylation Reactions
Cross-Coupling Reactions between Heteroatom Nucleophiles and Halides – Making Carbon–Heteroatom Bonds
Triflates as Substrates for Palladium-Catalysed Reactions
Mechanisms of Palladium(0)-Catalysed Processes
Reactions Involving Electrophilic Palladation
Copper-Catalysed Amination
Selectivity
Chapter 5: Pyridines
Electrophilic Addition to Nitrogen
Electrophilic Substitution at Carbon
Nucleophilic Substitution
Nucleophilic Addition to Pyridinium Salts
C-metallated Pyridines
Palladium(0)-Catalysed Reactions
Oxidation and Reduction
Pericyclic Reactions
Alkyl and carboxylic acid Substituents
Oxygen Substituents
N-Oxides
Amine Substituents
Ring Synthesis – Disconnections
Synthesis of Pyridines from 1,5-dicarbonyl Compounds (1,2- and 1,6-bonds made)
Synthesis of Pyridines from an aldehyde, two Equivalents of a 1,3-dicarbonyl Compound and Ammonia (1,2-, 3,4-, 4,5-, and 1,6-bonds made)
Synthesis of Pyridines from 1,3-dicarbonyl Compounds and a C2N unit (3,4- and 1,6-bonds made)
Chapter 6: Diazines
Electrophilic Addition to Nitrogen
Electrophilic Substitution at Carbon
Nucleophilic Substitution
Radical Substitution
C-Metallated Diazines
Palladium(0)-Catalysed Reactions
Pericyclic Reactions
Oxygen Substituents (see also pages 48 and 164)
N-Oxides (see also page 49)
Amine Substtuents (see also pages 48 and 164)
Ring Synthesis – Disconnections
Synthesis of Pyridazines from 1,4-dicarbonyl Compounds (2,3- and 1,6-bonds made)
Synthesis of Pyrimidines from 1,3-dicarbonyl Compounds (3,4- and 1,6-bonds made)
Synthesis of Pyrazines from 1,2-dicarbonyl Compounds (4,5- and 1,6-bonds made)
Synthesis of Pyrazines from α-amino-carbonyl Compounds (1,2- and 4,5-bonds made)
Benzodiazines
Chapter 7: Quinolines and Isoquinolines
Electrophilic Addition to Nitrogen
Electrophilic Substitution at Carbon
Nucleophilic Substitution
Nucleophilic Addition to Quinolinium/Isoquinolinium Salts
Palladium(0)-Catalysed Reactions
Oxidation and Reduction
Alkyl Substituents
Oxygen Substituents
N-Oxides
Ring Synthesis – Disconnections
Synthesis of Quinolines from Anilines (1,2- and 4,4a-Bonds Made)
Synthesis of Quinolines from Ortho-Aminoaryl Ketones or Aldehydes (1,2- and 4,4a-bonds made)
Synthesis of Isoquinolines from 2-arylethamines (1,2- and 1,8a-Bonds Made)
Synthesis of Isoquinolines from Aryl-Aldehydes and an Aminoacetaldehyde Acetal (1,2- and 4,4a-Bonds Made)
Synthesis of Isoquinolines from Ortho-Alkynyl Aryl-Aldehydes or Corresponding Imines (2,3-Bond Made)
Chapter 8: Pyryliums, Benzopyryliums, Pyrones and Benzopyrones
Pyrylium Salts
Electrophiles
Nucleophilic Addition
Ring-Opening Reactions of 2H-Pyrans
Oxygen Substituents – Pyrones and Benzopyrones
Ring Synthesis of Pyryliums from 1,5-Diketones (1,2-Bond Made)
Ring Synthesis of 4-Pyrones from 1,3,5-Triketones (1,2-Bond Made)
Ring Synthesis of 2-Pyrones from 1,3-Keto-Aldehydes (1,2- and 4,5-Bonds Made)
Ring Synthesis of 1-Benzopyryliums, Coumarins and Chromones
Chapter 9: Pyrroles
Electrophilic Substitution at Carbon
N-Deprotonation and N-Metallated Pyrroles
C-Metallated Pyrroles
Palladium(0)-Catalysed Reactions
Oxidation and Reduction
Pericyclic Reactions
Reactivity of Side-Chain Substituents
The ‘Pigments of Life’
Ring Synthesis – Disconnections
Synthesis of Pyrroles from 1,4-Dicarbonyl Compounds (1,2- and 1,5-Bonds Made)
Synthesis of Pyrroles from α-Amino-Ketones (1,2- and 3,4-Bonds Made)
Synthesis of Pyrroles Using Isocyanides (2,3- and 4,5-Bonds Made)
Chapter 10: Indoles
Electrophilic Substitution at Carbon
N-Deprotonation and N-Metallated Indoles
C-Metallated Indoles
Palladium(0)-Catalysed Reactions
Oxidation and Reduction
Pericyclic Reactions
Reactivity of Side-Chain Substituents
Oxygen Substituents
Ring Synthesis – Disconnections
Synthesis of Indoles from Arylhydrazones (1,2- and 3,3a-Bonds Made)
Synthesis of Indoles from ortho-Nitrotoluenes (1,2- and 2,3-Bonds Made)
Synthesis of Indoles from ortho-Aminoaryl Alkynes (1,2-Bond Made)
Synthesis of Indoles from ortho-Alkylaryl Isocyanides (2,3-Bond Made)
Synthesis of Indoles from ortho-Acyl Anilides (2,3-Bond Made)
Synthesis of Isatins from Anilines (1,2- and 3,3a-Bonds Made)
Synthesis of Oxindoles from Anilines (1,2- and 3,3a-Bonds Made)
Synthesis of Indoxyls from Anthranilic Acids (1,2- and 2,3-Bonds Made)
Azaindoles
Chapter 11: Furans and Thiophenes
Electrophilic Substitution at Carbon
Palladium(0)-Catalysed Reactions
Oxidation and Reduction
Pericyclic Reactions
Oxygen Substituents
Ring Synthesis – Disconnections
Synthesis of Furans and Thiophenes from 1,4-Dicarbonyl Compounds (1,2-Bond Made)
Chapter 12: 1,2-Azoles and 1,3-Azoles
Introduction
Electrophilic Addition to N
Electrophilic Substitution at C
Nucleophilic Substitution of Halogen
N-Deprotonation and N-Metallated Imidazoles and Pyrazoles
C-Metallated N-Substituted Imidazoles and Pyrazoles, and C-Metallated Thiazoles and Isothiazoles
C-Deprotonation of Oxazoles and Isoxazoles
Palladium(0)-Catalysed Reactions
1,3-Azolium Ylides
Reductions
Pericyclic Reactions
Oxygen and Amine Substituents
1,3-Azoles Ring Synthesis – Disconnections
Synthesis of Thiazoles and Imidazoles from α-Halo-Ketones (1,5- and 3,4-Bonds Made)
Synthesis of 1,3-Azoles from 1,4-Dicarbonyl Compounds (1,2- and 1,5-Bonds Made)
Synthesis of 1,3-Azoles Using Tosylmethyl Isocyanide (1,2- and 4,5-Bonds Made)
Synthesis of 1,3-Azoles Via Dehydrogenation
1,2-Azoles Ring Synthesis – Disconnections
Synthesis of Pyrazoles and Isoxazoles from 1,3-Dicarbonyl Compounds (1,5- and 2,3-Bonds Made)
Synthesis of Isoxazoles and Pyrazoles from Alkynes (1,5- and 3, 4-Bonds Made)
Synthesis of Isothiazoles from β-Amino α,β-Unsaturated Carbonyl Compounds (1,2-Bond Made)
Chapter 13: Purines
Electrophilic Addition to Nitrogen
Electrophilic Substitution at Carbon
N-Deprotonation and N-Metallated Purines
Oxidation
Nucleophilic Substitution
C-Metallated Purines by Direct Deprotonation or Halogen–metal Exchange
Palladium(0)-Catalysed Reactions
Purines with Oxygen and Amine Substituents
Ring Synthesis – Disconnections
Synthesis of Purines from 4,5-Diaminopyrimidines (7,8- and 8,9-Bonds Made)
Synthesis of Purines from 5-Aminoimidazole-4-Carboxamide (1,2- and 2,3-Bonds Made)
‘One-Step Syntheses’
Chapter 14: Heterocycles with More than Two Heteroatoms: Higher Azoles (5-Membered) and Higher Azines (6-Membered)
Introduction
Higher Azoles Containing Nitrogen as the Only Ring Heteroatom: Triazoles, Tetrazole and Pentazole
Benzotriazole
Higher Azoles also Containing Ring Sulfur or Oxygen: Oxa- and Thiadiazoles
Higher Azines
Chapter 15: Heterocycles with Ring-Junction Nitrogen (Bridgehead Nitrogen)
Introduction
Indolizine
Azaindolizines
Synthesis of Indolizines and Azaindolizines
Quinoliziniums and Quinolizinones
Heteropyrrolizines (Pyrrolizines Containing Additional Heteroatoms)
Cyclazines
Chapter 16: Non-Aromatic Heterocycles
Introduction
Three-Membered Rings
Four-Membered Rings
Five- and Six-Membered Rings
Ring Synthesis
Chapter 17: Heterocycles in Nature
Heterocyclic α-Amino Acids and Related Substances
Heterocyclic Vitamins – Co-Enzymes
Porphobilinogen and the ‘Pigments of Life’
Heterocyclic Secondary Metabolites
Chapter 18: Heterocycles in Medicine
Medicinal Chemistry – How Drugs Function
Drug Discovery
Drug Development
The Neurotransmitters
Histamine
Acetylcholine (ACh)
Anticholinesterase Agents
5-Hydroxytryptamine (5-HT) (Serotonin)
Adrenaline and Noradrenaline
Other Significant Cardiovascular Drugs
Drugs Acting Specifically On the CNS
Other Enzyme Inhibitors
Anti-Infective Agents
Antiparasitic Drugs
Antibacterial Drugs
Antiviral Drugs
Anticancer Drugs
Photochemotherapy
Chapter 19: Applications and Occurrences of Heterocycles in Everyday Life
Introduction
Dyes and Pigments (see also Page 190)
Polymers
Pesticides
Explosives
Food and Drink
Heterocyclic Chemistry of Cooking
Natural and Synthetic Food Colours
Flavours and Fragrances (F&F)
Toxins
Electrical and Electronic
Index
Other Titles Available in the Chemistry at a Glance Series:
Steroid Chemistry at a Glance
Daniel Lednicer
ISBN: 978-0-470-66084-3
Chemical Thermodynamics at a Glance
H. Donald Brooke Jenkins
ISBN: 978-1-4051-3997-7
Environmental Chemistry at a Glance
Ian Pulford, Hugh Flowers
ISBN: 978-1-4051-3532-0
Natural Product Chemistry at a Glance
Stephen P. Stanforth
ISBN: 978-1-4051-4562-6
The Periodic Table at a Glance
Mike Beckett, Andy Platt
ISBN: 978-1-4051-3299-2
Chemical Calculations at a Glance
Paul Yates
ISBN: 978-1-4051-1871-2
Organic Chemistry at a Glance
Laurence M. Harwood, John E. McKendrick, Roger Whitehead
ISBN: 978-0-86542-782-2
Stereochemistry at a Glance
Jason Eames, Josephine M Peach
ISBN: 978-0-632-05375-9
Reaction Mechanisms at a Glance: A Stepwise Approach to Problem-Solving in Organic Chemistry
Mark G. Moloney
ISBN: 978-0-632-05002-4

This edition first published 2013
© 2013 John Wiley & Sons, Ltd
Registered office
John Wiley & Sons Ltd, The Atrium, Southern Gate, Chichester, West Sussex, PO19 8SQ, United Kingdom
For details of our global editorial offices, for customer services and for information about how to apply for permission to reuse the copyright material in this book please see our website at www.wiley.com.
The right of the author to be identified as the author of this work has been asserted in accordance with the Copyright, Designs and Patents Act 1988.
All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, except as permitted by the UK Copyright, Designs and Patents Act 1988, without the prior permission of the publisher.
Wiley also publishes its books in a variety of electronic formats. Some content that appears in print may not be available in electronic books.
Designations used by companies to distinguish their products are often claimed as trademarks. All brand names and product names used in this book are trade names, service marks, trademarks or registered trademarks of their respective owners. The publisher is not associated with any product or vendor mentioned in this book. This publication is designed to provide accurate and authoritative information in regard to the subject matter covered. It is sold on the understanding that the publisher is not engaged in rendering professional services. If professional advice or other expert assistance is required, the services of a competent professional should be sought.
The publisher and the author make no representations or warranties with respect to the accuracy or completeness of the contents of this work and specifically disclaim all warranties, including without limitation any implied warranties of fitness for a particular purpose. This work is sold with the understanding that the publisher is not engaged in rendering professional services. The advice and strategies contained herein may not be suitable for every situation. In view of ongoing research, equipment modifications, changes in governmental regulations, and the constant flow of information relating to the use of experimental reagents, equipment, and devices, the reader is urged to review and evaluate the information provided in the package insert or instructions for each chemical, piece of equipment, reagent, or device for, among other things, any changes in the instructions or indication of usage and for added warnings and precautions. The fact that an organization or Website is referred to in this work as a citation and/or a potential source of further information does not mean that the author or the publisher endorses the information the organization or Website may provide or recommendations it may make. Further, readers should be aware that Internet Websites listed in this work may have changed or disappeared between when this work was written and when it is read. No warranty may be created or extended by any promotional statements for this work. Neither the publisher nor the author shall be liable for any damages arising herefrom.
Library of Congress Cataloging-in-Publication Data
Joule, J. A. (John Arthur)
Heterocyclic chemistry at a glance / John Joule, Keith Mills. – 2nd ed.
p. cm.
Includes index.
ISBN 978-0-470-97122-2 (cloth) – ISBN 978-0-470-97121-5 (pbk.) 1. Heterocyclic chemistry–Textbooks 2. Chemistry–Textbooks. I. Mills, K. (Keith) II. Title.
QD400.J594 2012
547'.59–dc23
2012016201
A catalogue record for this book is available from the British Library.
Cloth ISBN: 9780470971222
Paper ISBN: 9780470971215
Answers to the exercises are available on the accompanying website http://booksupport.wiley.com
Biography
John Arthur Joule was born in Harrogate, Yorkshire, England, but grew up and attended school in Llandudno, North Wales, going on to study for BSc, MSc, and PhD (1961; with George F. Smith) degrees at The University of Manchester. Following post-doctoral periods in Princeton (Richard K. Hill) and Stanford (Carl Djerassi) he joined the academic staff of The University of Manchester where he served for 41 years, retiring and being appointed Professor Emeritus in 2004. Sabbatical periods were spent at the University of Ibadan, Nigeria, Johns Hopkins Medical School, Department of Pharmacology and Experimental Therapeutics, and the University of Maryland, Baltimore County. He was William Evans Visiting Fellow at Otago University, New Zealand.
Dr. Joule has taught many courses on heterocyclic chemistry to industry and academe in the UK and elsewhere. He is currently Associate Editor for Tetrahedron Letters, Scientific Editor for Arkivoc, and Co-Editor of the annual Progress in Heterocyclic Chemistry.
Keith Mills was born in Barnsley, Yorkshire, England and attended Barnsley Grammar School, going on to study for BSc, MSc and PhD (1971; with John Joule) degrees at The University of Manchester.
Following post-doctoral periods at Columbia (Gilbert Stork) and Imperial College (Derek Barton/Philip Magnus), he joined Allen and Hanburys (part of the Glaxo Group) at Ware and later Stevenage (finally as part of GSK), working in Medicinal Chemistry and Development Chemistry departments for a total of 25 years. During this time he spent a secondment at Glaxo, Verona. Since leaving GSK he has been an independent consultant to small pharmaceutical companies.
Dr. Mills has worked in several areas of medicine and many areas of organic chemistry, but with particular emphasis on heterocyclic chemistry and the applications of transition metal-catalysed reactions.
Heterocyclic Chemistry was first published in 1972, written by George Smith and John Joule, followed by a second edition in 1978. The third edition (Joule, Mills and Smith) was written in 1995 and, after the death of George Smith, a fourth edition (Joule and Mills) appeared in 2000 and a fifth edition in 2010. The first edition of Heterocyclic Chemistry at a Glance was published in 2007.
Abbreviations
| Ac |
acetyl [CH3C=O], thus AcOH = ethanoic (acetic) acid; Ac2O = ethanoic anhydride (acetic anhydride) |
| anti |
on the opposite side (antonym of syn) |
| aq |
aqueous – the reaction mixture contains water |
| Ar |
general designation for a benzenoid aromatic group |
| [bmim][BF4] |
1-n-butyl-3-methylimidazolium tetrafluoroborate (an ionic liquid) |
| BINAP |
2,2′-bis(diphenylphosphino)-1,1′-binaphthyl – ligand for palladium(0) |
| Bn |
benzyl [PhCH2] – N-protecting group; removed by hydrogenolysis over Pd |
| Boc |
t-butyloxycarbonyl [t-BuOCO] – protecting group; removed with acid |
| Bom |
benzyloxymethyl [PhCH2OCH2] – protecting group; removed by hydrogenolysis over Pd |
| Bt |
benzotriazol-1-yl (structure page 136) |
| Bz |
benzoyl [PhCO] as in OBz, a benzoate |
| c |
cyclo as in c-C6H11 = cyclohexyl |
| c. |
concentrated, as in c. H2SO4 = concentrated sulfuric acid |
| cat |
catalyst – reagent not consumed in the reaction – usually, in the case of metal catalysts, e.g. Pd, used in sub-stoichiometric quantities – 1–5 mol% |
| Cbz |
benzyloxycarbonyl [PhCH2OCO] – protecting group; removed by hydrogenolysis |
| CDI |
1,1′-carbonyldiimidazole [(C3H3N2)2C=O] – peptide coupling reagent |
| Cy |
cyclohexyl [c-C6H11] |
| dba |
trans,trans-dibenzylideneacetone [PhCH=CHCOCH=CHPh] – ligand for palladium(0) |
| DCC |
dicyclohexylcarbodiimide [c-C6H11N=C=Nc-C6H11] – for coupling acid and amine to give amide |
| DDQ |
2,3-dichloro-5,6-dicyano-1,4-benzoquinone – oxidant, often used for dehydrogenation |
| DMAP |
4-dimethylaminopyridine [4-Me2NC5H4N] – nucleophilic catalyst |
| DME |
1,2-dimethoxyethane [MeO(CH2)2OMe] – ethereal solvent |
| DMF |
dimethylformamide [Me2NCH=O] – dipolar aprotic solvent |
| DMFDMA |
dimethylformamide dimethyl acetal [Me2NCH(OMe)2] |
| DMSO |
dimethylsulfoxide [Me2S=O] – dipolar aprotic solvent |
| dppb |
1,4-bis(diphenylphosphino)butane [Ph2P(CH2)4PPh2] – ligand for palladium(0) |
| dppe |
1,2-bis(diphenylphosphino)ethane [Ph2P(CH2)2PPh2] – ligand for palladium(0) |
| dppf |
1,1′-bis(diphenylphosphino)ferrocene [(Ph2PC5H4)2Fe] – ligand for palladium(0) |
| dppp |
1,3-bis(diphenylphosphino)propane [Ph2P(CH2)3PPh2] – ligand for palladium(0) |
| ee |
enantiomeric excess – a measure of the efficiency of an asymmetric synthesis |
| El+ |
general designation for a positively charged electrophile |
| Et |
ethyl [CH3CH2] |
| f. |
fuming, as in f. HNO3 = fuming nitric acid |
| 2-Fur |
furan-2-yl [C4H3O] |
| GABA |
γ-aminobutyric acid (4-aminobutanoic acid) [H2N(CH2)3CO2H] |
| Hal |
general designation for a halogen |
| Het |
general designation for a heteroaryl group |
| i-Pr |
isopropyl [Me2CH] |
| LDA |
lithium di-isopropylamide [LiN(i-Pr)2] – hindered strong base |
| LiTMP |
lithium 2,2,6,6-tetramethylpiperidide [LiN(C(Me)2(CH2)3C(Me)2] – hindered non-nucleophilic strong base |
| Me |
methyl [CH3] |
| Ms |
methanesulfonyl (mesyl) [MeSO2] – protecting group for azole nitrogen |
| NaHMDS |
sodium bis(trimethylsilyl)amide [sodium hexamethyldisilazide] [NaN(SiMe3)2] – hindered non-nucleophilic strong base |
| NBS |
N-bromosuccinimide [C4H4BrNO2] – brominating agent |
| NCS |
N-chlorosuccinimide [C4H4ClNO2] – chlorinating agent |
| NMP |
N-methylpyrrolidin-2-one (1-methylpyrrolidin-2-one) [C5H9NO] – dipolar aprotic solvent |
| n-Bu |
normal butyl [CH3(CH2)3] |
| n-Pr |
normal propyl [CH3CH2CH2] |
| Nu- |
general designation for a negatively charged nucleophile |
| Ph |
phenyl [C6H5] |
| PMB |
p-methoxybenzyl [4-MeOC6H4CH2] |
| Pr |
see i-Pr and n-Pr |
| 2-Py; 3-Py; 4-Py |
pyridin-2-yl; pyridin-3-yl; pyridin-4-yl [C5H4N] |
| R |
general designation for an alkyl group |
| o-Tol |
ortho-tolyl (2-methylphenyl) [C7H7] |
| p-Tol |
para-tolyl (4-methylphenyl) [C7H7] |
| rt |
room temperature (ca. 20 °C) |
| SelectfluorTM |
1-(chloromethyl)-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane tetrafluoroborate – electrophilic fluorinating agent |
| SEM |
trimethylsilylethoxymethyl [Me3Si(CH2)2OCH2] – protecting group; removed with fluoride |
| SES |
trimethylsilylethanesulfonyl [Me3Si(CH2)2SO2] – N-protecting group; removed with fluoride |
| SPhos |
2-dicyclohexylphosphino-2′,6′-dimethoxy-1,1′-biphenyl – ligand for palladium(0) |
| syn |
on the same side (antonym of anti) |
| TBDMS |
t-butyldimethylsilyl [t-Bu(CH3)2Si] – bulky silyl protecting group |
| t-Bu |
tertiary butyl [(CH3)3C] |
| Tf |
trifluoromethanesulfonyl [CF3SO2], thus TfO- = triflate [CF3SO3-] – triflate is a good leaving group |
| THF |
tetrahydrofuran – common ethereal solvent for dry reactions at low temperature |
| THP |
tetrahydropyran-2-yl [C5H9O] – protecting group; removed with aqueous acid |
| TIPS |
tri-isopropylsilyl [Si(i-Pr)3] – protecting group for nitrogen or oxygen |
| TIPB |
1,3,5-tri-iso-propylbenzene – inert high-boiling solvent |
| TMSCl |
trimethylsilyl chloride (chlorotrimethylsilane) [Me3SiCl] – O- and N-trimethylsilylating reagent |
| Tol |
same as p-Tol |
| TosMIC |
tosylmethyl isocyanide [TolSO2CH2N+KC-] |
| Ts |
p-toluenesulfonyl (tosyl) [p-TolSO2] – Ts is a good protecting group for azole nitrogen and Ts- can be a leaving group (para-toluensulfinate) |
| Tr |
trityl (triphenylmethyl) [Ph3C] – N-protecting group; removed with acid |
| TTF |
tetrathiafulvalene (C6H4S4) |
| X |
general designation for halogen (or in palladium(0) chemistry, sometimes OTf) |
Introduction to Second Edition
The material in this book comprises an introduction to, and summary of, the most important ideas and principles of heterocyclic chemistry. We have attempted to encapsulate everything that a non-specialist, or beginning student, would need to know of the subject. At the same time, we believe that this book will serve as a good starting point for further, more extensive study of the subject.
This Second Edition has been expanded by 50% compared with the First Edition (2007), allowing us to include more examples and illustrations, and exercises at the ends of the chapters (with answers available online at http://booksupport.wiley.com). The other significant difference to the First Edition is the use of colour in the schemes (for details, see below).
We now have three supplementary chapters dealing with the occurrence and significance of heterocycles in the world at large: Chapters 17 and 18 deal with ‘Heterocycles in Nature’ and ‘Heterocycles in Medicine’; Chapter 19 discusses major significant heterocyclic involvements in dyes and pigments, polymers, pesticides, explosives, food and drink, and electronics.
The book is mainly concerned with aromatic heterocycles though we also include a short discussion of non-aromatic heterocycles (Chapter 16). We deal with the characteristic reactivities of the most important heteroaromatic systems and the principal routes for their ring synthesis from non-heterocyclic precursors. Thus the chemistry of pyridines, pyridazines, pyrimidines, pyrazines, quinolines, isoquinolines, pyrylium and benzopyrylium cations, pyrroles, indoles, thiophenes, furans, imidazoles, oxazoles, thiazoles, pyrazoles, isoxazoles, isothiazoles, purines, heterocycles with more than two heteroatoms in the ring (for example triazoles and triazines) and heterocycles in which a heteroatom is located at a ring junction (for example pyrrolizines and indolizines) is covered (Chapters 5–15). The book starts with a discussion of nomenclature and structures of aromatic heterocycles (Chapters 1 and 2); then follows Chapter 3, which examines in detail the typical reactions of heterocycles, except for those involving palladium-catalysis, since these are considered separately in the following Chapter 4.
The book assumes a basic knowledge of organic chemistry such as one would expect of a student at the second year level of a UK Honours Chemistry course and thus would be suitable for second/third/fourth year undergraduate and post-graduate courses in UK Universities. It is also relevant that much Inorganic Chemistry relies on maintaining metals in various (often unusual) oxidation states by surrounding them with ligands and that these are very often heterocyclic, so choosing or designing appropriate heterocyclic ligands and then being able to synthesise them, is also an integral prerequisite of Inorganic Chemistry. With this book we also target students in other disciplines – Pharmacy, Pharmacology, Medicinal Chemistry – whose subjects require them to assimilate the basics of this particular area of organic chemistry. The vital importance of a proper understanding of heterocyclic chemistry for the study of biochemistry at the molecular level and for drug design and synthesis in medicinal chemistry, is emphasised in Chapters 17 and 18, ‘Heterocycles in Nature’ and ‘Heterocycles in Medicine’.
It is not the purpose of this book to provide guidance for the conduct of practical work: especially at the undergraduate level, all experimental work must be conducted under the supervision of an experienced teacher. For experimental details the reader must consult the original literature – many references to suitable, key papers can be found in our fuller exposition – Heterocyclic Chemistry, 5th Edition, Joule and Mills, Wiley, 2010. All the examples in Heterocyclic Chemistry at a Glance are taken from the literature and the vast majority proceed in good yields. In the reaction schemes, so that the reader can concentrate on the chemistry in question, we have simply shown that a particular compound will react with a particular reagent or reactant to give a product, and we have omitted practical details such as solvent, reaction time, yields, and most other details, except where their inclusion makes a didactical point. Where reactions were carried out at room temperature or with gentle warming or cooling, no comment is made. Where reactions were carried out with strong heating (e.g. reflux in a high-boiling solvent) the word ‘heat’ is used on the reaction arrow; for transformations carried out at very low temperature, this is specified on the reaction arrow. For some of the palladium-catalysed reactions we give full experimental conditions, to illustrate what is typical for cross-couplings.
In the reaction schemes, we have highlighted in red those parts of the products (or intermediates) where a change in structure or bonding has taken place. We hope that this both facilitates comprehension of the chemical processes that are occurring and quickly focuses the reader's attention on just those parts of the molecules where structural change has occurred. For example, in the first reaction below, only changes at the pyridine nitrogen are involved; in the second example, the introduced bromine resulting from the substitution, and its new bond to the heterocycle, are highlighted. The exception to this policy is in palladium-catalysed cross-coupling processes where the functional groups in each of the coupling partners, as well as the new bond formed, are coloured red, as shown in the third example below.
Finally we acknowledge the crucial advice, support and encouragement from staff at Wiley, in bringing this project to fruition, in particular Paul Deards and Sarah Tilley. Mrs Joyce Dowle is thanked for her helpful comments during the preparation of Chapter 19 and Judith Egan-Shuttler for her careful copy editing.
Further Reading
This book can act only as an introduction to heterocyclic chemistry and does not include references to original literature, or to the many reviews that are available. For further study and to go more deeply into the topics covered in this book we recommend, as a first port-of-call, our textbook Heterocyclic Chemistry [1] in which there are a host of leading references to the original literature and appropriate reviews.
The premier sources of regular reviews in this area are Advances in Heterocyclic Chemistry [2] and Progress in Heterocyclic Chemistry [3] and the principles of heterocyclic nomenclature are set out in one review [4] in the former series. The journal, Heterocycles, also carries many useful reviews specifically in the heterocyclic area. As its title implies, an exhaustive coverage of the area is provided in the three parts of Comprehensive Heterocyclic Chemistry (CHC), original (1984), and its two updates (1996 and 2008) [5]. Note: The three parts must be read together – the later parts update but do not repeat the earlier material. The Handbook of Heterocyclic Chemistry [6] that accompanies CHC encapsulates the key information from the series in a single volume. There is a comprehensive compilation of heterocyclic data and facts: the still-continuing and still-growing series of monographs [7] dealing with particular heterocyclic systems, edited originally by Arnold Weissberger, and latterly by Edward C. Taylor and Peter Wipf, is a vital source of information and reviews for all those working with heterocyclic compounds. The ‘Science of Synthesis’ series contains authoritative discussions on the synthesis of heterocycles, organized in a hierarchical system [8]; volumes 9–17, published over the period 2000–2008, discuss aromatic heterocycles.
For further reading relating in particular to Chapters 17, 18 and 19, we recommend Heterocycles in Life and Society [9], Introduction to Enzyme and Coenzyme Chemistry [10], Nucleic Acids in Chemistry and Biology [11], The Alkaloids; Chemistry and Biology [12], Comprehensive Medicinal Chemistry II [13], Molecules and Medicine [14], Goodman and Gilman's The Pharmacological Basis of Therapeutics [15], The Chemistry of Explosives [16], Food. The Chemistry of its Components [17], Perfumes: the Guide [18], Handbook of Conducting Polymers [19], Handbook of Oligo- and Polythiophenes [20], Tetrathiafulvalenes, Oligoacenenes, and their Buckminsterfullerene Derivatives: the Bricks and Mortar of Organic Electronics [21].
References
1. Heterocyclic Chemistry, 5th edition, Joule, J. A. and Mills, K., Wiley, 2010; ISBN 978-1-405-19365-8 (cloth); 978-1-405-13300-5 (paper).
2. Advances in Heterocyclic Chemistry, 1963–2012 Volumes 1–105.
3. Progress in Heterocyclic Chemistry, 1989–2012, Volumes 1–24.
4. ‘The Nomenclature of Heterocycles’, McNaught, A. D., Advances in Heterocyclic Chemistry, 1976, 20, 175.
5. Comprehensive Heterocyclic Chemistry. The Structure, Reactions, Synthesis, and Uses of Heterocyclic Compounds, Eds. Katritzky, A. R. and Rees, C. W., Volumes 1–8, Pergamon Press, Oxford, 1984; Comprehensive Heterocyclic Chemistry II. A review of the literature 1982–1995, Eds. Katritzky, A. R., Rees, C. W., and Scriven, E. F. V., Volmes 1–11, Pergamon Press, 1996; Comprehensive Heterocyclic Chemistry III. A review of the literature 1995–2007, Eds. Katritzky, A. R., Ramsden, C. A., and Scriven, E. F. V., and Taylor, R. J. K., Volumes 1–15, Elsevier, 2008.
6. Handbook of Heterocyclic Chemistry, 3rd edition, 2010, Katritzky, A. R., Ramsden, C. A., Joule, J. A., and Zhdankin, V. V., Elsevier, 2010.
7. The Chemistry of Heterocyclic Compounds, Series Eds. Weissberger, A., Wipf, P., and Taylor, E. C., Volumes. 1–64, Wiley-Interscience, 1950–2005.
8. Science of Synthesis, Volumes 9–17, ‘Hetarenes’, Thieme, 2000–2008.
9. Heterocycles in Life and Society. An Introduction to Heterocyclic Chemistry, Biochemistry and Applications, 2nd edition, Pozharskii, A. F., Soldatenkov, A. T., and Katritzky, A. R., Wiley 2011.
10. Introduction to Enzyme and Coenzyme Chemistry, 2nd edition, Bugg, T., Blackwell, 2004.
11. Nucleic Acids in Chemistry and Biology, Eds. Blackburn, G. M., Gait, M. J., and Loakes, D., Royal Society of Chemistry, 2006.
12. The Alkaloids; Chemistry and Biology, Volumes 1–70, original Eds. Manske, R. H. F. and Holmes, H. L., Ed. Cordell, G. A., 1950–2011.
13. Comprehensive Medicinal Chemistry II, Eds. Triggle, D. and Taylor, J., Elsevier, 2006.
14. Molecules and Medicine, Corey, E. J., Czakó, B., and Kürti, L., Wiley, 2007. This is a useful general discussion from a chemical/biochemical viewpoint of major drugs of all structural types.
15. Goodman & Gilman's The Pharmacological Basis of Therapeutics, 11th edition, Eds. Brunton, L. L., Lazo, J. S., and Parker, K. L., McGraw-Hill, 2005. This is the standard textbook, which is subject to frequent revision.
16. The Chemistry of Explosives, 3rd edition, Akhavan, J., Royal Society of Chemistry, 2011.
17. Food. The Chemistry of its Components, 5th edition, Coultate, T., Royal Society of Chemistry, 2009.
18. Perfumes: the Guide, Turin, L. and Sanchez, T., Profile Books, 2008.
19. Handbook of Conducting Polymers, 2nd edition, Eds. Skotheim, T. A. and Reynolds, J. R., Taylor & Francis, 2007.
20. Handbook of Oligo- and Polythiophenes, Ed. Fichou, D., Wiley, 1998.
21. ‘Tetrathiafulvalenes, Oligoacenenes, and their Buckminsterfullerene Derivatives: the Bricks and Mortar of Organic Electronics’, Bendikov, M., Wudl, F., and Perepichka, D. F., Chemical Reviews, 2004, 104, 4891.
Chapter 1
Heterocyclic Nomenclature
A selection of the structures, names and standard numbering of the more common heteroaromatic systems and some common non-aromatic heterocycles, are shown in this chapter. The aromatic heterocycles are grouped into those with six-membered rings and those with five-membered rings. The names of six-membered aromatic heterocycles that contain nitrogen generally end in ‘ine’, though note that ‘purine’ is the name for a very important bicyclic system which, has both a six- and a five-membered nitrogen-containing heterocycle. Five-membered heterocycles containing nitrogen generally end with ‘ole’. Note the use of italic ‘H’ in a name such as ‘9H-purine’ to designate the location of an N-hydrogen in a system in which, by tautomerism, the hydrogen could reside on another nitrogen (e.g. N-7 in the case of purine). Names such as ‘pyridine’, ‘pyrrole’ and ‘thiophene’ are the original, and now standard, names for these heterocycles; names such as ‘1,2,4-triazine’ for a six-membered ring with three nitrogens located as indicated by the numbers, are more logically systematic.
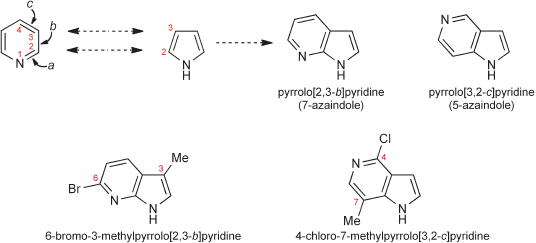
A detailed discussion of the systematic rules for naming polycyclic systems in which several aromatic or heteroaromatic rings are fused together, is beyond the scope of this book, however, two simple examples will serve to illustrate the principles. In the name ‘pyrrolo[2,3-b]pyridine’, the numbers signify the positions of the first named heterocycle, numbered as if it were a separate entity, which are the points of ring fusion; the italic letter, ‘b’ in this case, designates the side of the second named heterocycle to which the other ring is fused, the lettering deriving from the numbering of that heterocycle as a separate entity, that is, side a is between atoms 1 and 2, side b is that between atoms 2 and 3, and so on. Actually, this particular heterocycle is more often referred to as ‘7-azaindole’ – note the use of the prefix ‘aza’ to denote the replacement of a ring carbon by nitrogen. Similarly, ‘5-azaindole’ is systematically called ‘pyrrolo[3,2-c]pyridine’ – note that the order of the numbers ‘3,2-’ arises because the first atom of the pyrrole encountered in counting round from the pyridine nitrogen to determine the side of fusion, and thus the label ‘c’, is C-3 of the pyrrole unit. The numbering of a bi- or polycyclic system as a whole is generated from a series of rules concerned with the orientation of the rings and the positions of the nitrogen(s), but we do not deal with these here – the overall numbering for these two systems is shown for two substituted examples.
A device that is useful in discussions of reactivity is the designation of positions as ‘α’, ‘β’ or ‘γ’. For example, the 2- and the 6-positions in pyridine are equivalent in reactivity terms, so to make discussion of such reactivity clearer, each of these positions is referred to as an ‘α-position’. Comparable use of α and β is made in describing reactivity in five-membered systems. These useful designations are shown on some of the structures. Note that carbons at angular positions do not have a separate number but are designated using the number of the preceding atom followed by ‘a’ – as illustrated for quinoline.
Six-Membered Aromatic Heterocycles
Five-Membered Aromatic Heterocycles
Non-Aromatic Heterocycles
Small-Ring Heterocycles








