

Table of Contents
Series Page
Title Page
Copyright
Acknowledgements and Dedication
List of Contributors
Preface–Introduction
Diagnostic Electron Microscopy
The purpose and use of TEM
The aim and purpose of this book
Chapter 1: Renal Disease
1.1 The Role of Transmission Electron Microscopy (TEM) in Renal Diagnostics
1.2 Ultrastructural Evaluation and Interpretation
1.3 The Normal Glomerulus
1.4 Ultrastructural Diagnostic Features
1.5 The Ultrastructural Pathology of the Major Glomerular Diseases
References
Chapter 2: Transplant Renal Biopsies
2.1 Introduction
2.2 The Transplant Renal Biopsy
2.3 Indications for Electron Microscopy of Transplant Kidney
References
Chapter 3: Electron Microscopy in Skeletal Muscle Pathology
3.1 Introduction
3.2 Normal Muscle
3.3 Pathological Changes
References
Chapter 4: The Diagnostic Electron Microscopy of Nerve
4.1 Introduction
4.2 Tissue Processing
4.3 Normal Nerve Ultrastructure
4.4 Pathological Ultrastructural Features
4.5 Artefact
4.6 Conclusions
References
Chapter 5: The Diagnostic Electron Microscopy of Tumours
5.1 Introduction
5.2 Principles and Procedures for Diagnosing Tumours by Electron Microscopy
5.3 Organelles and Groups of Cell Structures Defining Cellular Differentiation
References
Chapter 6: Microbial Ultrastructure
6.1 Introduction
6.2 Practical Guidance
6.3 Viruses
6.4 Current Use of EM in Virology
6.5 Viruses in Thin Sections of Cells or Tissues
6.6 Bacteria
6.7 Fungal Organisms
6.8 Microsporidia
6.9 Parasitic Protozoa
6.10 Examples of Non-enteric Protozoa
6.11 Parasitic Amoebae
6.12 Conclusions
Acknowledgements
References and Additional Reading
Chapter 7: The Contemporary Use of Electron Microscopy in the Diagnosis of Ciliary Disorders and Sperm Centriolar Abnormalities
7.1 Introduction
7.2 Ultrastructure of Motile Cilia
7.3 Genetics of PCD
7.4 Current Diagnostic Modalities
7.5 Clinical Features
7.6 Procurement and Assessment of Ciliated Specimens
7.7 Centriolar Sperm Abnormalities
7.8 Discussion
Acknowledgements
References
Chapter 8: Electron Microscopy as a Useful Tool in the Diagnosis of Lysosomal Storage Diseases
8.1 Introduction
8.2 Morphological Findings
8.3 Conclusion
References
Chapter 9: Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy (CADASIL)
9.1 Introduction
9.2 Diagnostic Strategies—Comparative Specificity and Sensitivity
9.3 Diagnosis by TEM
References
Chapter 10: Diagnosis of Platelet Disorders by Electron Microscopy
10.1 Introduction
10.2 TEM Preparation of Platelets
10.3 Whole-Mount EM Preparation of Platelets
10.4 EM Preparation of Bone Marrow
10.5 Pre-embed Immunogold Labelling of Von Willibrand Factor in Platelets
10.6 Ultrastructural Features of Platelets
10.7 Normal Platelets
10.8 Grey Platelet Syndrome
10.9 Arthrogryposis, Renal Dysfunction and Cholestasis Syndrome
10.10 Jacobsen Syndrome
10.11 Hermansky–Pudlak Syndrome, Chediak–Higashi Syndrome and Other Dense-Granule Deficiencies
10.12 Type 2B von Willebrand Disease and Platelet-Type von Willebrand Disease
References
Chapter 11: Diagnosis of Congenital Dyserythropoietic Anaemia Types I and II by Transmission Electron Microscopy
11.1 Introduction
11.2 Preparation of Bone Marrow and General Observation Protocol
11.3 CDA Type I
11.4 CDA Type II
Acknowledgements
References
Chapter 12: Ehlers–Danlos Syndrome
12.1 Introduction
12.2 Collagen Fibrils
12.3 Elastic Fibers
12.4 Nonfibrous Stroma and Granulo-Filamentous Deposits
12.5 Connective Tissue Disorders
References
Chapter 13: Electron Microscopy in Occupational and Environmental Lung Disease
13.1 Introduction
13.2 Asbestos
13.3 Hypersensitivity Pneumonitis and Sarcoidosis
13.4 Silicosis
13.5 Silicate Pneumoconiosis
13.6 Metal-Induced Diseases
13.7 Rare-Earth Pneumoconiosis
13.8 Miscellaneous Disorders
References
Chapter 14: General Tissue Preparation Methods
14.1 Introduction
14.2 Tissue Collection and Dissection
14.3 Tissue Processing
14.4 Tissue Sectioning
References
Chapter 15: Ultrastructural Pathology Today—Paradigm Change and the Impact of Microwave Technology and Telemicroscopy
15.1 Diagnostic Electron Microscopy and Paradigm Shift in Pathology
15.2 Standardised and Automated Conventional Tissue Processing
15.3 Microwave-Assisted Sample Preparation
15.4 Cyberspace for Telepathology via the Internet
15.5 Conclusions and Future Prospects
Acknowledgements
References
Chapter 16: Electron Microscopy Methods in Virology
16.1 Biological Safety Precautions
16.2 Collection of Specimens
16.3 Preparation of Faeces, Vomitus or Urine Samples
16.4 Viruses in Skin Lesions
16.5 Reagents and Methods
16.6 Coated Grids
16.7 Important Elements in the Negative Staining Procedure
16.8 TEM Examination
16.9 Immunoelectron Microscopy
16.10 Thin Sectioning of Virus-Infected Cells or Tissues
16.11 Virology Quality Assurance (QA) Procedures
Acknowledgements
References
Chapter 17: Digital Imaging for Diagnostic Transmission Electron Microscopy
17.1 Introduction
17.2 Camera History
17.3 The Pixel Dilemma
17.4 Camera Positioning
17.5 Resolution
17.6 Fibre Coupled or Lens Coupled?
17.7 Sensitivity, Noise and Dynamic Range
17.8 CCD Chip Type (Full Frame or Interline)
17.9 Binning and Frame Rate
17.10 Software
17.11 Choosing the Right Camera
References
Chapter 18: Uncertainty of Measurement
18.1 Introduction
18.2 Purpose
18.3 Factors That Influence Quantitative Measurements
18.4 How to Calculate the UM
18.5 Worked Examples
18.6 Conclusion
References
Index
Current and future titles in the Royal Microscopical Society –John Wiley Series
Principles and Practice of Variable Pressure/Environmental Scanning Electron Microscopy (VP-ESEM)
Debbie Stokes
Aberration-Corrected Analytical Electron Microscopy
Edited by Rik Brydson
Diagnostic Electron Microscopy—A Practical Guide to Interpretation and Technique
Edited by John W. Stirling, Alan Curry & Brian Eyden
Low Voltage Electron Microscopy: Principles and Applications
Edited by David C. Bell & Natasha Erdman
Atlas of Images and Spectra for Electron Microscopists
Edited by Ursel Bangert
Understanding Practical Light Microscopy
Jeremy Sanderson
Focused Ion Beam Instrumentation: Techniques and Applications
Dudley Finch & Alexander Buxbaum
Electron Beam-Specimen Interactions and Applications in Microscopy
Budhika Mendis

This edition first published 2013
© 2013 John Wiley & Sons Ltd.
Registered office
John Wiley & Sons Ltd, The Atrium, Southern Gate, Chichester, West Sussex, PO19 8SQ, United Kingdom
For details of our global editorial offices, for customer services and for information about how to apply for permission to reuse the copyright material in this book please see our website at www.wiley.com.
The right of the author to be identified as the author of this work has been asserted in accordance with the Copyright, Designs and Patents Act 1988.
All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, except as permitted by the UK Copyright, Designs and Patents Act 1988, without the prior permission of the publisher.
Wiley also publishes its books in a variety of electronic formats. Some content that appears in print may not be available in electronic books.
Designations used by companies to distinguish their products are often claimed as trademarks. All brand names and product names used in this book are trade names, service marks, trademarks or registered trademarks of their respective owners. The publisher is not associated with any product or vendor mentioned in this book. This publication is designed to provide accurate and authoritative information in regard to the subject matter covered. It is sold on the understanding that the publisher is not engaged in rendering professional services. If professional advice or other expert assistance is required, the services of a competent professional should be sought.
The publisher and the author make no representations or warranties with respect to the accuracy or completeness of the contents of this work and specifically disclaim all warranties, including without limitation any implied warranties of fitness for a particular purpose. This work is sold with the understanding that the publisher is not engaged in rendering professional services. The advice and strategies contained herein may not be suitable for every situation. In view of ongoing research, equipment modifications, changes in governmental regulations, and the constant flow of information relating to the use of experimental reagents, equipment, and devices, the reader is urged to review and evaluate the information provided in the package insert or instructions for each chemical, piece of equipment, reagent, or device for, among other things, any changes in the instructions or indication of usage and for added warnings and precautions. The fact that an organization or Website is referred to in this work as a citation and/or a potential source of further information does not mean that the author or the publisher endorses the information the organization or Website may provide or recommendations it may make. Further, readers should be aware that Internet Websites listed in this work may have changed or disappeared between when this work was written and when it is read. No warranty may be created or extended by any promotional statements for this work. Neither the publisher nor the author shall be liable for any damages arising herefrom.
Library of Congress Cataloging-in-Publication Data
Diagnostic electron microscopy : a practical guide to interpretation and technique / edited by John W. Stirling, Alan Curry, and Brian Eyden.
p. ; cm.
Includes bibliographical references and index.
ISBN 978-1-119-97399-7 (cloth)
I. Stirling, John W. II. Curry, Alan. III. Eyden, Brian.
[DNLM: 1. Diagnostic Imaging–methods. 2. Microscopy, Electron, Transmission. WN 180]
616.07′54—dc23
2012027835
A catalogue record for this book is available from the British Library.
ISBN: 978-1-119-97399-7
Acknowledgements and Dedication
All three editors wish to thank the many individuals who have helped to make this volume possible. Firstly, they would like to express their appreciation to all the authors for their hard work and generosity in sharing their professional experience, as well as all the ‘behind-the-scenes’ staff and colleagues without whom this book could not have been produced.
John Stirling thanks the staff of the Centre for Ultrastructural Pathology, SA Pathology, Adelaide, for their support and photographic contributions—especially Alvis Jaunzems and Jeffrey Swift—and Dr Sophia Otto of the Department of Surgical Pathology, SA Pathology, for her advice and for proofreading.
Alan Curry acknowledges the contributions to his work of the pathologists, particularly Dr Helen Denley and Dr Lorna McWilliam, and technical staff of the Manchester Royal Infirmary, as well as two inspirational organisations—the Public Health Laboratory Service Electron Microscopy network and the Manchester Electron Microscope Society.
Brian Eyden wishes to thank all of the Pathology Department staff at the Christie NHS Foundation Trust (Manchester), without whose technical and light microscopic input the interpretation of tumour ultrastructure would be compromised, if not, in some instances, impossible.
Secondly, the editors wish to recognise the support and encouragement of their families in this endeavour. John Stirling thanks his partner, Jill, and expresses a special appreciation of his teachers and mentors, particularly Alec Macfarlane who helped him achieve his dream of a career in biology and Andrew Dorey who introduced him to electron microscopy and the wonders of cell ultrastructure. Alan Curry thanks his wife, Collette (particularly for her exceptional computer skills), and Brian Eyden thanks his wife, Freda, for understanding the needs of a writing scientist.
Finally, the editors dedicate this book to diagnostic electron microscopists—wherever they may be—who continue to make uncertain diagnoses more precise as a result of their labours, which, in turn, help clinicians to treat their patients better, the ultimate purpose of our work.
List of Contributors
Preface–Introduction
Science progresses as a result of a variety of factors. Critical to progress, however, is the invention and availability of appropriate tools and techniques that can completely transform our ability to investigate and understand the world around us—without such tools our ability to investigate even basic phenomena would be severely restricted. One such ‘transformational’ technology is the electron microscope. Although transmission electron microscopy (TEM) is now taken for granted, its application to the biological and medical sciences in the late 1950s and early 1960s ranks as one of the single most important factors that has impacted on our knowledge in biology and medicine. The resolving power of the transmission electron microscope (∼0.2 nm as compared with the light microscope with a resolution of ∼200 nm) made two important things possible for the first time, these being the visualisation of: (1) cell organelles and cytoplasmic structures at the macromolecular level (both useful indicators of cell differentiation) and; (2) viruses and microorganisms in general. Thus, TEM gave us new fundamental insights into cell structure and function, histogenesis and differentiation, and, following from this, our understanding of disease and disease processes.
TEM was quickly taken up as a diagnostic tool. In the clinical setting, electron microscopy has been used to improve diagnostic precision and confidence in many fields, including renal disease, neuromuscular disease, microbiology (particularly virology), tumour pathology, skin diseases, industrial diseases, haematology, metabolic storage diseases and conditions involving abnormalities of cilia and sperm. A number of encyclopaedic atlases of normal and pathological tissues quickly followed the introduction of electron microscopy and the medical literature contains many articles describing diagnostic applications of TEM in a wide range of conditions and specialist areas. Diagnostic TEM reached a zenith during the 1980s; however, since then, the introduction of new methodologies (particularly molecular techniques and affinity labelling systems) has reduced the need for TEM, particularly in tumour diagnosis. Despite this, TEM continues to play a significant and important role in pathology, and techniques continue to develop and improve. For example, the introduction of microwave processing and digital cameras has transformed tissue processing and screening so that ‘same-day’ reporting is easily achieved.
The purpose of TEM is to diagnose disease based on the ultrastructural features of the tissue. These features include:
In general, the use of TEM will be predetermined either as a stand-alone protocol (e.g., CADASIL) or as part of a broad integrated diagnostic strategy (e.g., renal biopsies). However, TEM can also be applied on an ad hoc basis whenever there is a chance it will give an improved diagnosis (and therefore better patient care). The general criteria indicating the use of TEM may be summarised simply as follows:
The prime aim and purpose of this book is to summarise the current interpretational applications of TEM in diagnostic pathology. In this respect, we have not attempted to reproduce previous encyclopaedic texts but to provide what we regard as a working guide to the main, or most useful, applications of the technique given the limited space available in a text of this size. In addition, we have also included practical topics of concern to laboratory scientists, including brief guides to traditional tissue and microbiological preparation techniques, microwave processing, digital imaging and measurement uncertainty.
Chapter 1
Renal Disease
The ultrastructural examination of renal biopsies has made a significant contribution to our understanding of renal disease and is fundamental to accurate diagnosis. For overall tissue evaluation, light microscopy (LM), immunolabelling and transmission electron microscopy (TEM) are generally combined as an integrated protocol. LM is used to make an assessment of overall tissue morphology and to identify the major pathological processes present. Immunolabelling (preferably using immunofluorescence or by the immunoperoxidase technique) is used to determine the composition and location of glomerular immune deposits. Local practices vary, but an antibody panel can contain antibodies directed against IgG, IgA, IgM, complement (C3, C1q and sometimes C4), κ and λ light chains and albumin. TEM can play a major role when LM and immunolabelling findings are normal, only mildly atypical or equivocal and difficult to interpret, particularly in respect to conditions where there may be similar LM or immunolabelling findings. Thus, the technique is particularly useful in the setting of familial disease where the structural abnormalities in the glomerular basement membrane (GBM) cannot be resolved by LM (e.g. Alport's syndrome). TEM can also provide critical information not revealed by the other methodologies to identify underlying primary disease and unexpected concomitant disease. Similarly with immunolabelling, the full classification and staging of deposits require ultrastructural analysis. Some transplant biopsies can also benefit from ultrastructural evaluation (see Chapter 2); however, TEM rarely contributes to the diagnosis of tubular, vascular or interstitial disease. Overall, ultrastructural screening is essential; it can change the diagnosis in ∼25% of cases and provides ‘useful’ information in ∼66% of cases (Pearson et al., 1994; Elhefnawy, 2011).
Examination of glomeruli (and other areas, if necessary) should be thorough and systematic with all components being evaluated for possibly significant features or changes. During screening, a range of representative images should be taken. These should include low-power images to show overall glomerular morphology, plus a representative selection of higher power images to show the specific and critical diagnostic features. In some instances, it may also be important to show that certain features are, in fact, absent (e.g. deposits) or normal (e.g. foot processes). The principal elements that should be examined are (i) the location, size and morphology of immune-related deposits and other inclusions; (ii) the thickness, overall morphology and texture of the GBM; (iii) the size and morphology of the mesangial matrix and (iv) the number and morphology of the cellular components of the glomerulus (Stirling et al., 2000). Sclerotic glomeruli should be avoided, and only well-preserved functional (or significantly functional) glomeruli should be examined. It is also important to ensure that the glomeruli screened are representative of the LM findings: this means that, ideally, the choice of glomeruli to be screened (from semithin sections) should be done in collaboration with the reporting pathologist. Finally, it should be stressed that screening should be unbiased, although some knowledge of the pathology and immunolabelling results may be useful if the features expected are minor or uncommon. The vascular pole should be avoided during ultrastructural evaluation as it may contain misleading nonpathologic deposits, and likewise Bowman's capsule which has no real diagnostic value, although the presence of crescents can be confirmed.
Following evaluation, representative images and findings should be communicated to the reporting pathologist, the latter verbally or in a concise written report. If the initial evaluation does not correspond with the LM evaluation (e.g. the electron microscopy (EM) samples only a tiny fraction of the available tissue), then the specimen should be re-examined or additional glomeruli observed to increase diagnostic confidence.
A critical question is ‘How many glomeruli should be examined, and for how long?’ Unfortunately, there is no definitive answer to this dilemma except to say that enough tissue should be examined to answer the diagnostic question posed and to ensure that no additional or unexpected pathology is present. A single glomerulus (or even part of one) may be adequate in respect to diffuse disease and/or when the glomerulus screened is typical of the disease process identified by LM. In contrast, several glomeruli, or possibly glomeruli from different blocks, may be required to capture the full range of pathological changes in focal disease. Perhaps the final word on this issue is to say that the tissue must be screened thoroughly; it is bad practice to stop screening once the features that were expected have been located because additional findings that affect the accuracy of the diagnosis may be missed.
The glomerulus (Figure 1.1) is composed of a tuft of branching capillaries that originate from the afferent arteriole at the vascular pole to form a series of lobules (segments) that ultimately rejoin at the vascular pole and exit the glomerulus via the efferent arteriole. At the core of each lobule is the mesangium which supports the capillary loops; capillary loops are lined by endothelial cells (Figure 1.1). The mesangial matrix principally consists of collagen IV and is populated by mesangial cells (usually 1–3 in normal mesangium) plus a small number of immune-competent cells and rare transient cells of the monocyte–macrophage lineage (Sterzel et al., 1982). The entire capillary tuft is enclosed within Bowman's capsule, the inner aspect of which is lined by a thin layer of epithelial cells (the parietal epithelial cells); a second inner population of epithelial cells (the visceral epithelial cells or podocytes) is closely associated with the capillary tufts, and extensions of these cells form the foot processes (pedicels) that cover the outer aspect of the capillary walls (Figure 1.1). The podocytes are the sole source of the collagen IV α3, α4 and α5 subtypes that form the bulk of the GBM (Abrahamson et al., 2009), and the foot processes play a major role in ultrafiltration and the maintenance of the filtration barrier. As a result, podocyte dysfunction plays a major role in a wide range of glomerular diseases (Wiggins, 2007; Haraldsson, Nystrom and Deen, 2008). Opposite the vascular pole, Bowman's capsule is continuous with the proximal tubule which drains filtrate from the glomerulus (the urinary pole). Overall, filtration is said to be a function of size, shape and charge selection, although the nature and contribution of charge selection are debated (Harvey et al., 2007; Haraldsson, Nystrom and Deen, 2008; Goldberg et al., 2009). The capillary wall as a whole is responsible for the filtration process, and it appears that the capillary endothelium, the GBM and the podocyte foot processes must all be intact for normal filtration to occur (Patrakka and Tryggvason, 2010).
Figure 1.1 Detail of a normal glomerulus. The capillary loops are supported by the mesangium (M). Mesangial cells with nuclei (MC); capillary lumens (L); urinary space (U); podocyte (P) (epithelial cell) and foot processes (FP). Here, the overall width of Overall, the glomerular basement membrane (GBM) averages ∼380 nm in width. Loops are lined with fenestrated endothelial cells (E). Bar = 5 μm.

The GBM (Figure 1.1) is made of three layers: (i) the lamina rara interna, the electron-lucent layer immediately adjacent to the endothelium; (ii) the lamina densa, the central layer and (iii) the lamina rara externa, the outer electron-lucent area immediately adjacent to the foot processes. The lamina densa makes up the bulk of the GBM and is its main structural element; it has a felt-like fibrillar construction, and knowledge of its molecular makeup is helpful in understanding and interpreting familial and autoimmune disease. The principal component is collagen IV, which consists of six subtypes (α1–α6) (Patrakka and Tryggvason, 2010). In the developing kidney, the GBM is initially formed of the α1 and α2 subtypes with the α3, α4 and α5 subtypes forming later (the additional subtype, α6, is restricted to Bowman's capsule and some tubular basement membranes) (Harvey et al., 1998; Miner, 1998). In the mature kidney, the α1 and α2 subtypes are restricted to a narrow band immediately adjacent to the capillary endothelium; the α3, α4 and α5 subtypes form the remaining bulk of the GBM (extending out to the foot processes). The core of the mesangial matrix is composed of the α1 and α2 subtypes (continuous with the inner aspect of the GBM), while the outer peripheral layer is made up of the α3, α4 and α5 subtypes (continuous with the outer layer of the GBM) (Butkowski et al., 1989; Harvey et al., 1998; Miner, 1998). The α3, α4 and α5 subtypes are essential for the maintenance of normal glomerular function, and mutations in the genes for these subtypes are responsible for the various forms of membrane-related hereditary nephritis. The structural abnormalities of the GBM in hereditary disease are caused by the absence of the α3 and α5 subtypes, because without either of these, the membrane fails to form correctly (Kalluri et al., 1997; LeBleu et al., 2010; Miller et al., 2010). The α3 subtype has been identified as the Goodpasture epitope (Saus et al., 1988). However, it appears that both the α3 and α5 subtypes are targeted in anti-GBM disease, while in Alport's post-transplantation nephritis, only the α5 subtype is involved (Pedchenko et al., 2010).
Immune-related material accumulates as discrete or linear deposits of finely granular electron-dense material within or adjacent to the GBM and/or mesangium in several diseases. Deposits may also be ‘organised’ as tubules and fibrils of various diameters, as crystals and as whorls with a fingerprint-like appearance (Herrera and Turbat-Herrera, 2010). The identity and content of specific deposits vary and must be confirmed by immunolabelling.
Note that scattered deposits may sometimes be an incidental finding with no obvious pathological or diagnostic relevance. Approximately 4–16% of normal individuals have mesangial IgA deposits (without IgG or C3) (Coppo, Feehally and Glassock, 2010), and small numbers of discrete deposits are occasionally seen in individuals with naturally high levels of antigenic challenge.
Subepithelial deposits are finely granular, medium-density deposits located on the outer surface of the GBM and the mesangium. Foot processes that lie over the surface of the deposit are generally effaced.
These are finely granular, medium-density deposits that lie completely within the lamina densa. The material may be uniform in appearance, or patchy and irregular. Resorption of deposits results in irregular electron-lucent areas surrounded by thickened GBM; badly damaged membrane may become laminated and similar in appearance to the ‘basket weave’ pattern seen in Alport's syndrome. Seen typically in stage III membranous glomerulonephritis (Figure 1.12).
These are linear or plaque-like, finely granular, medium-density deposits located between the inner aspect of the GBM and the capillary endothelium. Large deposits may be visible by LM as nodular hyaline ‘thrombi’ or as ‘wire-loop’ capillary wall thickening. Seen typically in MCGN type I (Figure 1.16).
Many normal and pathological fibrils are found in the glomerulus, and the deposition of proteins, such as monoclonal immunoglobulins or their light-chain or heavy-chain subunits, can produce several glomerular diseases (see reviews by Furness, 2004; Basnayake et al., 2011). The accurate identification of fibrils can be problematic, and distinguishing between normal and pathological types may require the correlation of ultrastructural, immunolabelling and LM-staining characteristics (Table 1.1). A number of algorithms have been published to aid in the diagnosis of renal diseases containing organised deposits (Figure 1.2) (see Ivanyi and Degrell, 2004; Herrera and Turbat-Herrera, 2010).
Figure 1.2 Algorithm for diagnosis of organised deposits. An algorithm to aid in the diagnosis of diseases with organised deposits (based on Ivanyi and Degrell, 2004; Herrera and Turbat-Herrera, 2010). Measurements given are fibril and tubule diameters (see Table 1.1 for source references). This strategy may be combined with silver methenamine staining to further define matrix-derived components (Herrera and Turbat-Herrera, 2010).
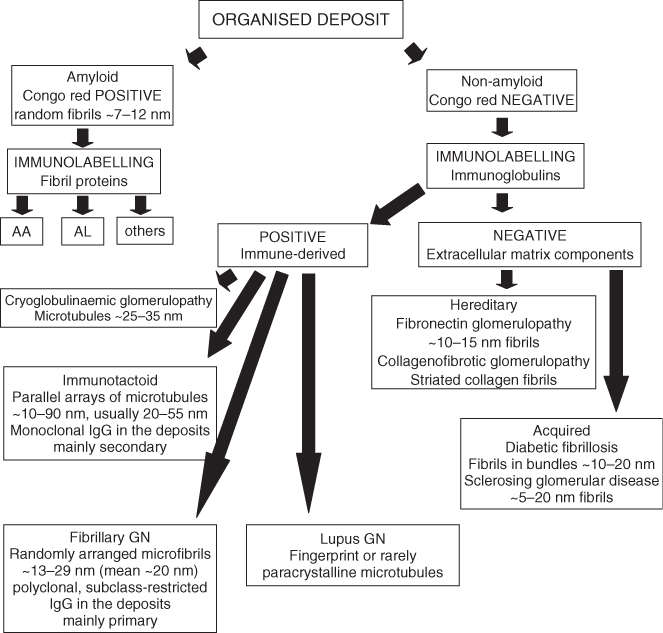
Table 1.1 Characteristics of immune and non-immune glomerular fibrils.
| Fibril type | Characteristics |
| Amyloid (Figure 1.22) | Transmission electron microscopy (TEM) characteristics |
| Extracellular nonbranching fibrils | |
| Random orientation, occasionally in parallel arrays | |
| Fibrils may appear to penetrate, or flow (cascade) from, adjacent cells | |
| Distribution diffuse or focal; includes GBM | |
| Diameter: ∼7–10 nm (Ghadially, 1988) or ∼8–12 nm (Rosenstock et al., 2003) | |
| Light microscopy (LM) characteristics | |
| Periodic acid–Schiff (PAS) negative; non-argyrophilic | |
| Congo red positive (also Sirius red and Thioflavin T and S positive in fresh tissue) | |
| Positive Congo red reaction requires a reasonable amount of amyloid to be present. Sections should be 8–10 μm thick. Apple green birefringence under polarised light (Vowles, 2008). | |
| Immunolabelling | |
| Negative for immunoglobulins, complement components, fibrinogen and albumin. Composition variable. Immunolabelling may be used to identify precursor proteins in order to identify amyloid type (Vowles, 2008). | |
| ‘Immune’ fibrils (fibrillary glomerulonephritis) (Figure 1.23) | TEM characteristics |
| Extracellular nonbranching fibrils | |
| Random, sometimes parallel orientation | |
| Distribution diffuse; includes GBM | |
| Diameter: 13–29 nm (mean 20.1 nm ±0.4) (Rosenstock et al., 2003) or 15–25 nm (Herrera and Turbat-Herrera, 2010) | |
| LM characteristics | |
| Glassy and weakly eosinophilic; weakly PAS positive; grey-purple on trichrome stain; non-argyrophilic (Rosenstock et al., 2003) | |
| PAS–methenamine–silver: weak (reticular) staining | |
| Congo red negative | |
| Immunolabelling | |
| Smudgy, ribbon-like to granular staining for IgG, C3 and κ and λ light chains (sometimes with light-chain restriction) in mesangium and peripheral capillary walls. IgG4 is dominant in most cases, but rarely IgG1 (Herrera and Turbat-Herrera, 2010). | |
| Immunotactoid glomerulopathy (‘immune’ tubules) (Figure 1.24) | TEM characteristics |
| Extracellular nonbranching tubules | |
| Orientation random, or in parallel arrays; sometimes in a background matrix (Herrera and Turbat-Herrera, 2010 | |
| Distribution diffuse; includes mesangium and GBM | |
| Diameter: 20–55 nm (mean 38.2 nm ± 5.7) (Rosenstock et al., 2003) or 10–90 nm (commonly more than 30 nm) (Herrera and Turbat-Herrera, 2010) | |
| LM characteristics | |
| Silver stains negative | |
| Immunolabelling | |
| Variable, IgG and C3 in mesangium and peripheral capillary walls with a granular or pseudolinear pattern; IgA, IgM and C1q variable or negative; light-chain restriction (κ rather than λ) in some cases (Herrera and Turbat-Herrera, 2010) | |
| Microfibrils | TEM characteristics |
| Extracellular nonbranching fibrils | |
| Parallel, ‘bundled’ or sometimes random orientation | |
| Normal microfibrillar glomerular components include (i) collagen fibrils ∼30 nm in diameter and greater; (ii) ‘large’ fibrils ∼18–20 nm in diameter; (iii) ‘small’ fibrils ∼10 nm in diameter and (iv) ‘thin filaments’ ∼3–5 nm in diameter (Coleman and Seymour, 1992). | |
| Nonspecific fibrils ∼12 nm in diameter may be found in several conditions, especially sclerosing glomerular diseases (Hsu and Churg, 1979; Kronz, Nue and Nadasdy, 1998). | |
| 12 nm diameter non-immune fibrils are a major component of Kimmelstiel–Wilson nodules (Figure 1.8) (Yasuda et al., 1992). | |
| LM characteristics | |
| Generally PAS and PAS–methenamine–silver strongly positive | |
| Congo red negative | |
| Immunolabelling | |
| Negative or nonspecific (Kronz, Nue and Nadasdy, 1998) |
Amyloid is composed of insoluble fine fibrils made up of low-molecular-weight proteins of various types in a β-pleated sheet conformation (Dember, 2006; Vowles, 2008). Amyloid deposits may form anywhere in the glomerulus (and other tissues) as extracellular nonbranching fibrils of indeterminate length and without cross-striations (Table 1.1; Figure 1.22). Immunofluorescence can sometimes indicate the possibility of amyloid deposition (dull green colouration), but this can be difficult to differentiate from diabetic changes (increase in matrix).
Both fibrils and tubules of immune-related material may be found in glomeruli; they may be randomly orientated or organised in parallel arrays. Reported diameters for both fibrils and tubules vary greatly, presumably because of their individual physiochemical makeup, the range of cases sampled and variability in laboratory processing regimes (Table 1.1). Fingerprint deposits (parallel or linear arrays of fibrils—usually curved in a fingerprint-like pattern; Figure 1.20) may also be found, particularly in systemic lupus erythematosus (SLE) and cryoglobulinaemia. There is some dispute as to whether diseases featuring immune fibrils (fibrillary glomerulonephritis; Figure 1.23) and those featuring tubules (immunotactoid glomerulopathy; Figure 1.24) should be treated together or separately (Schwartz, Korbet and Lewis, 2002; Ivanyi and Degrell, 2004; Herrera and Turbat-Herrera, 2010). Here, they are treated as separate entities as there are important clinical differences between them, that is, immunotactoid is normally associated with a lymphoproliferative disorder, but typically, fibrillary glomerulonephritis is not.
A variety of nonspecific fibrils that may be confused with immune deposits have been found in glomeruli (see Herrera and Turbat-Herrera, 2010; and review by Coleman and Seymour, 1992). Some normal matrix components may be more prominent in pathological conditions. Mesangial microfibrils may be particularly well developed in MCGN and diabetic glomerulosclerosis (Table 1.1). Fibrillar collagen is found within the GBM in nail–patella syndrome (Coleman and Seymour, 1992).
Several nonspecific inclusions such as microparticles (small electron-dense granules), fibrils and membrane-like material may be found within the GBM and mesangial matrix—mostly with doubtful or unknown diagnostic significance. Most common are accumulations of spherical particles and vesicles (often referred to as inclusions or virus-like particles): these may be found in the mesangium but are often seen in a subepithelial location, especially in the angle (or ‘notch’) between two adjoining capillary loops. Microparticles are sometimes seen in areas of laminated GBM in Alport's syndrome (Figure 1.5); similar granules are also found in thickened loops in diabetic sclerosis. Local accumulations of moderately electron-dense material similar to immune-related deposits may be observed, most commonly in a subendothelial location. In the absence of positive immunolabelling, these may relate to the insudation of plasma proteins and/or the development of ‘fibrin’ caps.
Fibrin may be present in numerous diseases in almost any location in the glomerulus. It may be found as irregular, angular or needle-like accumulations of amorphous material of medium electron density, or it may show cross-striations with a characteristic periodicity. The periodicity of normal fibrin is reported as 22.5 nm (Standeven, Ariëns and Grant, 2005); however, periodicities of ∼19–35 nm are reported in pathological tissues in general (Ghadially, 1988). Fibrin is easily distinguished from fibrillar collagen which has an axial banding periodicity of 64–68 nm.
Tubuloreticular bodies (TRBs) are small clusters of fine anastomosing tubules that arise within the cisternae of the rough endoplasmic reticulum; in the glomerulus, they are most commonly found in endothelial cells (Figure 1.18b). The formation of TRBs has been linked to α- and ß-interferon activity (Hammar et al., 1992). In renal disease, TRBs are most commonly associated with SLE (Figure 1.18b) (and collagen vascular diseases in general), with viral infections (especially HIV/AIDS and hepatitis B) and as a result of α-interferon treatment (Hammar et al., 1992; Haas et al., 2000; Haas, 2007; Yang et al., 2009b). However, TRBs are not specific to these conditions as they have also been found in renal transplants and have been linked to diabetes, lymphoma and Helicobacter pylori infection (Yang et al., 2009b). The presence of TRBs in idiopathic conditions can act as an indicator of underlying systemic disease, and their presence should prompt additional investigations (Yang et al., 2009b).
Reported measurements for adult GBM vary greatly, but mean widths generally fall in the range of 300–400 nm (Figure 1.1) (see Coleman et al., 1986; review by Dische, 1992; Bonsib, 2007). The GBM of children is reported as being slightly thinner up to age 9 (Morita et al., 1988). A review by Marquez et al. (1999) found that in addition to the variability in values reported for GBM width in adults, there are conflicting data regarding age- and sex-related differences: some studies indicate that the GBM in males and females is similar, but in others, males are said to have slightly thicker GBM than females. Similarly, some studies suggest that the GBM continues to increase in width with age, whereas others do not. Overall, this variation is presumed to reflect the type of tissue used to obtain ‘normal’ data, the differing morphometric techniques employed and the variation inherent in tissue processing and choice of reagents (Marquez et al., 1999; Edwards et al., 2009). With these variations in mind, it is clear that statements about GBM width must be treated with caution. For measurements to be meaningful, a set of normal values must be established based on local processing methodology.
The principal pathological change seen in the mesangium is enlargement of the extracellular matrix, a process that may lead to the obliteration of part or all of the glomerulus (sclerosis). Matrix expansion may be accompanied by an increase in cellularity, immune-related deposits and several nonspecific inclusions such as microfibrils and granular and/or vesicular material. Rarely, the mesangium may be dissolved or attenuated (mesangiolysis) so that the capillary loops are able to fuse and expand (Morita and Churg, 1983).
Endothelial swelling and hypercellularity may occur in many conditions. Significant numbers of TRBs may be found in several conditions and may contribute to the identification and understanding of underlying pathology (Yang et al., 2009b).
In proteinuric diseases, the normal structure and arrangement of the podocyte foot processes are often lost: this occurs when the actin cytoskeleton is reorganised, and the processes merge to form a continuous or semicontinuous cytoplasmic layer (foot process effacement, obliteration or fusion) (D'Agati, 2008; Haraldsson, Nystrom and Deen, 2008). Numerous cytoplasmic inclusions are found in podocytes, most notably lysosomes, lipid vesicles and protein droplets. Abnormal lysosomes may be present because of an inherited storage disorder such as Fabry's disease or as a result of drug treatment (e.g. amiodarone and gold therapy). Podocytes may develop numerous long microvilli in a process known as microvillous transformation (Figure 1.3).
Figure 1.3 Minimal change disease. Foot processes are almost completely effaced (foot process effacement), (FPE). The podocyte (P) contains several vesicles, some of which contain lipid (V); there is also significant microvillous transformation (MV). The glomerular basement membrane (GBM) is slightly thin (190 nm in the thinnest areas). L = capillary lumen. Bar = 5 μm.
