

Table of Contents
Related Titles
Title Page
Copyright
Preface
Acknowledgment
Chapter 1: Classification of Explosives
1.1 Initiation Sensitivity
1.2 Size
1.3 Usage
1.4 Physical Form
Chapter 2: Explosive Science
2.1 Introduction
2.2 Initiation and Detonation
2.3 Propagation and Detonation
2.4 Reaction Chemistry in Explosives
References
Chapter 3: Ammonium Nitrate Explosives
3.1 Introduction
3.2 Design of Commercial Explosives
3.3 Tests
3.4 Assessment of Safety and Stability Characteristics
3.5 Summary
References
Chapter 4: Ammonium Nitrate and AN/FO
4.1 Introduction and History
4.2 Physical and Chemical Properties of Ammonium Nitrate
4.3 Manufacture of Ammonium Nitrate
4.4 Ammonium Nitrate Fuel Oil Explosives
References
Further Reading
Chapter 5: Slurries and Water Gels
5.1 Development
5.2 Design
5.3 Process Technology
5.4 Quality Checks
5.5 Process Hazards (Dust Explosions/Fire Hazards/Health Hazards)
5.6 Role of GG
5.7 Permissible Explosives
5.8 General Purpose Small-Diameter Explosives (GPSD)
5.9 Sensitizers
References
Further Reading
Chapter 6: Emulsion Explosives
6.1 Introduction
6.2 Concept of Emulsion Explosives
6.3 General Composition of Emulsion Explosives
6.4 Structure and Rheology
6.5 Composition and Theory of Emulsion Explosives
6.6 Manufacture
6.7 Quality Checks
6.8 Explosive Properties of Emulsion Matrix/Explosives
6.9 Permissible Emulsions
6.10 General Purpose Small-Diameter (GPSD) Emulsion Explosives
6.11 Bulk Emulsions
6.12 Heavy AN/FO
6.13 Packaged Large-Diameter Emulsion Explosives
References
Further Reading
Chapter 7: Research and Development
7.1 Areas of Interest
7.2 Development Work and Upscaling
7.3 Management of R&D
Chapter 8: Functional Safety during Manufacture of AN Explosives
8.1 Introduction – Personal View Point on Safety
8.2 Safety Considerations in AN Explosives
8.3 Explosion Hazards in Equipment
8.4 Concluding Remarks
References
Chapter 9: Economics of AN-Based Explosives
9.1 In Manufacture
9.2 In Applications
9.3 Blast Design
9.4 Influence of Explosives in Underground Mining
References
Chapter 10: Current Status and Concluding Remarks
Appendix A
Appendix B: Guidelines for Investigation of an Accident
B.1 Introduction
B.2 Detailed Inspection
B.3 Interviewing and Questioning
B.4 Collection of Samples
B.5 Examination of Witnesses
B.6 Examination of Dead/Injured
Index
Related Titles
Koch, Ernst-Christian
Metal-Fluorocarbon Based Energetic Materials
2012
ISBN: 978-3-527-32920-5
Lackner, M., Winter, F., Agarwal, A. K. (eds.)
Handbook of Combustion
2010
ISBN: 978-3-527-32449-1
Agrawal, J. P.
High Energy Materials
Propellants, Explosives and Pyrotechnics
2010
ISBN: 978-3-527-32610-5
Meyer, R., Köhler, J., Homburg, A.
Explosives
2007
ISBN: 978-3-527-31656-4
Agrawal, J. P., Hodgson, R.
Organic Chemistry of Explosives
2007
ISBN: 978-0-470-02967-1
Kubota, N.
Propellants and Explosives
Thermochemical Aspects of Combustion
2007
ISBN: 978-3-527-31424-9
Teipel, U. (ed.)
Energetic Materials
Particle Processing and Characterization
2005
ISBN: 978-3-527-30240-6

The Author
Dr. Erode G. Mahadevan
Technology Consultant
C-22 Vikrampuri Colony
Secunderabad 500009
India
All books published by Wiley-VCH are carefully produced. Nevertheless, authors, editors, and publisher do not warrant the information contained in these books, including this book, to be free of errors. Readers are advised to keep in mind that statements, data, illustrations, procedural details or other items may inadvertently be inaccurate.
Library of Congress Card No.: applied for
British Library Cataloguing-in-Publication Data
A catalogue record for this book is available from the British Library.
Bibliographic information published by the Deutsche Nationalbibliothek
The Deutsche Nationalbibliothek lists this publication in the Deutsche Nationalbibliografie; detailed bibliographic data are available on the Internet at <http://dnb.d-nb.de>.
© 2013 Wiley-VCH Verlag & Co. KGaA, Boschstr. 12, 69469 Weinheim, Germany
All rights reserved (including those of translation into other languages). No part of this book may be reproduced in any form — by photoprinting, microfilm, or any other means — nor transmitted or translated into a machine language without written permission from the publishers. Registered names, trademarks, etc. used in this book, even when not specifically marked as such, are not to be considered unprotected by law.
Print ISBN: 978-3-527-33028-7
ePDF ISBN: 978-3-527-64570-1
ePub ISBN: 978-3-527-64569-5
mobi ISBN: 978-3-527-64571-8
oBook ISBN: 978-3-527-64568-8
Preface
Ammonium nitrate (AN) explosives came into prominence in the last three decades (40 years) for civil applications as it provided a greater margin of safety to the manufacturer and end user as compared to the then popular nitroglycerine (NG) explosives. Due to rapid industrialization over the last 30 years, there has been a surge in mining and infrastructure activities which in turn has triggered high demand for civil explosives at all types of remote and tough locations. Mining methodology also underwent a change to cater to these requirements and huge open cast mines which need for their efficient operations large volumes of explosives delivered at mine site are operating in great numbers. Such enhanced requirements could only be satisfied by AN-based civil explosives which can be manufactured in high tonnages with a good margin of safety and low capital investment. Thus in many countries, manufacture and use of NG explosives were reduced drastically or abandoned, and AN explosives were used instead. Rapid development of AN-based explosives for all types of applications including underground gassy coal mines took place between 1970 and 1990 to fill in the void left by NG explosives and a number of patents appeared during this time. Over the years, however, the manufacture in industrial scale has settled down to fairly common practices and raw materials.
Despite the importance of the AN-based civil explosives today, there has not been much written and published about these explosives in detail perhaps because of their unglamorous nature as compared to military explosives and propellants. It is my intention to fill this gap by devoting the contents of this book exclusively to the technology of manufacture of AN civil explosives. This book will deal with three such products – AN/fuel oil explosives (AN/FO), slurry and water gel explosives, and emulsions explosives, in great detail as they comprise nearly 90% of the explosives used worldwide in civil sector.
Much has been published about the chemistry and science of explosives as well as test methods employed to determine their characteristics. It is my intention not to repeat these but mention only the most important aspects applicable to AN explosive under consideration here. On the other hand, the book will concentrate on providing valid data and sensible manufacturing guidelines based on the author's hands-on experience of more than 35 years in this field. There is no attempt here to bring into print any kind of proprietary information and “tricks of the trade” being practiced in the industry. The author hopes that the contents will benefit the persons engaged in the industry to have a better understanding of the role of the critical factors involved in manufacturing good explosives in a safe way. It is also the fond hope of the author that through this book young and fresh minds will get stimulated to take up research in this subject, which has been woefully very meager, and contribute toward better understanding of the basics leading to safer and hopefully cheaper products in keeping with current environmental conditions.
I feel the chapters describing the critical role of raw materials like guar gums, aluminum powders, emulsifiers, processing and packing technology, and optimum utilization of explosives energy in field applications will be of great interest to the reader. Strangely while the science of explosives includes aspects of importance from complex subjects like thermodynamics, colloid chemistry, powder metallurgy, mixing technology, and detonation physics, the commercial manufacture of AN explosives for civil application has reduced to a fairly low technology, high volume industry where know-how is supreme and know-why is of low importance. Hence there is no theoretical and mathematical approach of the subject in the book but attempt is made to demystify and simplify concepts of explosive phenomena so as to enable those performing routine jobs in this industry to understand and appreciate more their occupation, thereby deriving more intellectual satisfaction.
The contents of the book will be of interest to persons engaged in the civil explosives industry in all its aspects such as manufacturing, quality assurance and safety, scientists in research establishments, statutory authorities in the field of civil explosives, individual consultants to the explosive industry, managers in the mining industry, and so on. The contents of the book could be used to write up a production and safety manual as also for troubleshooting in existing operations. The blasting engineer may also be able to use its chapter on application to derive the maximum benefit from the use of the explosives.
The book, after exploring the evolution of these three types of explosives, will contain individual chapters dealing with science and technology, manufacturing, safety, and future R&D work needed.
Individual chapters are exclusive to the type of the explosive being discussed. The three major explosives dealt with are AN/FO, slurry/watergels, and emulsion explosives.
Individual chapters describe the following:
General topics of interest are contained in
Erode G. Mahadevan
Acknowledgment
It is not easy to write an acknowledgment for a book production as the number of people involved could be very many. The contents of my book are a mix of my own thinking and experience, but after stimulating discussions with various people connected with the global explosives industry and practical data collected over many years. But to my mind the motivation to stay and do research in this field was provided by the inspiring personality of Prof. T. Urbanskii with whom I came into contact while working in IDL industries, Hyderabad. The final impetus to write a book came through Dr. Martin Preuss of Wiley-VcH. I owe a lot to my family for their encouragement and support. Apart from these the various persons with whom I interacted in the industry at one time or the other inspired me to try and seek some answers to the phenomenon of explosives but special mention has to be made to the great working atmosphere provided by IDL Industries (now known as Gulf Oil India) where I spent a greater part of my career and gained hands-on knowledge in the field of explosives.
I am grateful to all the above for their role in motivating me to write a book primarily intended for the new entrants to the field of civil explosives as it stands today. Hopefully, I have succeeded in satisfying the readers of my book in whatever they expected from the contents.
I also wish to thank Dr. Martin Graf-Utzman and his staff for assisting me in finalizing this book.
Chapter 1
Classification of Explosives
Explosives are classified into different types and categories in various ways depending on their usage, sensitivity to initiation, and finished product packaging.
Figure 1.1 Types of initiation.
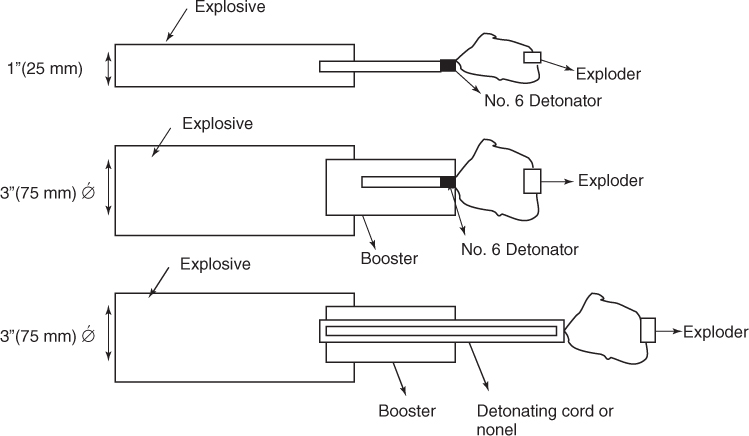
Explosives are further classified into primary, secondary, and tertiary explosives depending on its level of sensitivity to external stimuli. Standardized testing evaluates the sensitivity in terms of friction, impact, heat, shock and based on these results, explosives are classified accordingly.
Nitroglycerine (NG) is very sensitive and classified as a primary explosive. TNT/RDX/dynamites are secondary explosives. These are relatively safe for handling and can be handled in large-scale production plants with acceptable degree of safety. Ammonium nitrate (AN) explosives are the least sensitive and come in the tertiary explosives group. Even though they may have higher detonation velocities and pressure than NG explosives, they are much safer to produce in very large quantities.
The boosters are in turn set off by either detonator or by a coil of detonating cord wound over and through it (see Figure 1.1).
The explosives are also classified into general purpose and permissible categories.
Classification according to physical form of end product is as follows:
Any material which cannot be fully set off with a measurable velocity of detonation (VOD) either by detonator or by detonation is considered as “nonexplosive” in nature. However such nonexplosive material can be converted into an explosive by increasing its sensitivity.
Chapter 2
Explosive Science
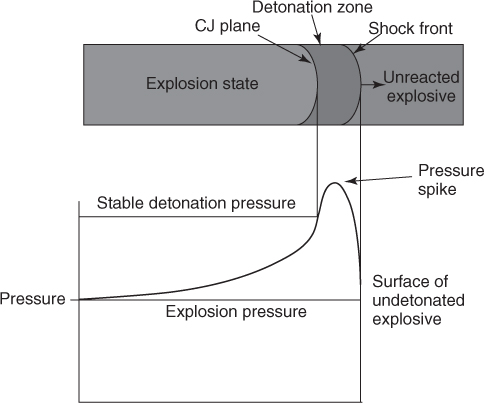
Explosives are known in practice as substances which work on the surroundings when they are set off. In the open area, their effectiveness is much less than under confinement because most of the work is done by expanding gases. Gas-producing event can be due to burning (deflagration) or explosion and detonation. One usually differentiates by the reaction velocities and pressures achieved in each of the phenomena. Thus while in deflagration, reaction velocities are much slower than velocity of sound and the pressures attained are in the range of bars. In detonation, the reaction velocity, which produces gas due to chemical reaction of the explosive with its own ingredients or air, exceeds the speed of sound in the material itself; thus there is a supersonic shock wave produced. The wavefront travels in advance of the release of expanding gases. The shock energy has a high peak pressure but is transient, whereas the gas energy is a longer lasting event though lower in peak pressure attained (see Figure 2.1) [1].
Figure 2.1 Schematic representations of zones and pressure variations along a detonating explosive charge.
Deflagration and fast-burning substances which still perform some amount of work through release of gas are classified as low explosives. Black powder is a typical example. Reaction velocities are normally in the range of 600 − 1000 m/s (see Figure 2.2) [1].
Figure 2.2 Pressure profiles for low and high explosives.
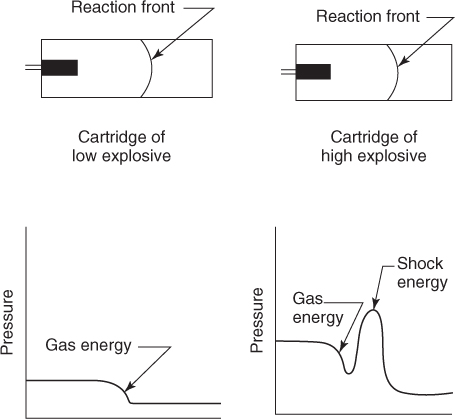
Velocity of detonation (VOD) are in excess of 1800 m/s. Most commercial explosives and especially the ammonium nitrate (AN) based belong to the high explosives category due to their high detonation and gas pressures.
Explosives can also be classified as homogeneous and heterogeneous. Usually primary and secondary explosives are present in the former, whereas in the latter tertiary explosives which are mixtures of chemicals are found.
In order to perform, explosives need to be initiated. The extent of initiation needed depends on the sensitively of the explosive. Tertiary explosives – AN-based explosives, can be initiated by either a detonator or a booster like PENTOLITE. It is worthwhile to understand the basics of the phenomenon of initiation because of its importance, it being the first step toward detonation which is the ultimate goal.
In the case of most commercially used AN-based explosives, there is no self-explosive ingredient added on and the detonation is based on the rapid decomposition of AN and its reaction with the surrounding materials. As mentioned earlier if the chemical reaction is completed within the shock velocities, then detonation has occurred. Theoretical treatment of the mechanism of initiation abounds in literature [2–7] but while there are variations, there is also unanimity that hotspots within the explosive are essential for initiation and propagation of the detonation wave [8].
The hotspot theory has been to a great extent supported by experimental data obtained while formulating and testing AN explosives. The initiation is achieved by compression of the unreacted chemical (AN) causing local shear failure and inelastic flow that creates hotspots, which in turn sustain the chemical reaction by supplying intense heat [9, 10] in excess of the losses that could arise due to side effects as the wave travels. Several mechanisms postulated for creation of the hotspots and causing initiation are adiabatic compressions of voids through (i) shock pressure wave, (ii) friction, (iii) shear, and (iv) deformation.
These are achieved by shock/mechanical impact of hot metallic fragments in practice when a detonator shell explodes inside a tertiary explosive. On the other hand boosters initiate through pressure wave compression. Difference in initiation capability of No. 6/No. 8 for some explosives is due to higher brisance of No. 8 detonator containing higher quantity of explosive and its ability to accelerate metal fragments. This depends on the base charge, higher density, particle size, and physical status.
However, all the above mechanism finally lead to a thermal event (increase in temperature), which accelerates the decomposition reaction into a runaway situation. The latest theory finding acceptance is that inelastic flow of the explosive under the influence of detonation wave from the initiation is able to create a lower stress required for easier initiation.
Propagation can be defined as the event where in an explosive the detonation process goes on till all the explosive material is consumed.
Propagation in a cylindrical charge of explosive is from the end of initiation to the other end. This is the most common event as most explosives are used in cylindrical shape of varying lengths and diameters. The word propagation itself means continuation and hence for an explosive to succeed initiation/detonation propagation is a must. The mechanism of propagation has also been the subject of study by many [11, 12].
Initiation per se does not ensure propagation. Attaining a steady-state detonation with a constant measurable velocity is a sure sign of propagation. For propagation to continue there must be continuity in the decomposition of the explosive till the end. This is achieved best when the losses due to conduction to the sides in a cylindrical charge is much less than the heat evolved as the wave travels through a cylindrical charge. There is net heat energy gain which accelerates the chemical reaction following Arrhenius equation. This heat generation is a self-sustaining multiplying situation as long as the heat transfer rate cannot keep pace with the exothermic heat produced by the reaction. Pressure and temperature rapidly increase leading to detonation in the direction of the shock wave. These conditions are especially critical in small diameter explosive charges.
From initiation to propagation to detonation completes the chain of events as far as the explosive is concerned. Most of the postulations and theoretical calculations were made in the period of 1950–1970 and correlation with experimental data attempted. The study was mainly confined to pure explosive compounds. A certain degree of confidence and uniformity in prediction of behavior was established. However heterogeneous mixtures such as ANFO/slurries/emulsions were studied later since these were backbone of commercial blasting operations. Studies while not carried in depth were sufficient to establish guidelines to determine optimum behavior in practice. AN/FO being the simplest explosive consisting of two or three components was examined at great length and useful parameters were established.
Study of the detonation phenomenon has shown that the term detonation can be applied to the event where an explosive on receiving an impulse undergoes rapid and continuous exothermic chemical reaction. If the rate of reaction exceeds the velocity of sound in the reacting material, a supersonic shock wave is established. This shock wave propagates and ends when all the explosive material has reacted. The shock wave also produces a sharp high pressure which peaks and subsides as it propagates. The shock wave being a compression wave also produces heat in the body of the explosive. Just behind the shock wave is the reaction zone where the chemical decomposition is taking place (see Figure 2.3). Further due to this decomposition and combination with oxygen, gaseous products are evolved and they rapidly expand due to heat. This rapid expansion of the products of reaction from materials making up the explosive is the major work force employed for deriving a heaving effect while blasting. The energy in a shock wave as estimated, calculated, and measured is different. Bubble energy for different explosives and in absolute terms could be 15–20% for the shock energy and 80% for Total energy. The partition of total energy between shock and bubble is also different for different explosives (see Table 2.2).
Figure 2.3 Structure of Detonation front [11].

The event of detonation and measurements thereof have shown that VOD is steady throughout and for the same explosive does not increase or decrease unless the diameter increases or decreases within limits. Where there is no further increase in VOD, however much the cylindrical cross section of the explosive increases; then it is termed as a state of ideal detonation. Conversely where the VOD is affected by the diameter of the explosive, nonideal detonation exists. The difference in diameter for these two states for most commercial explosives can be significant and has to be kept in mind during blasting operations.
Detonation has also thrown up the fact that behavior of explosive changes subject to its diameter. The smallest diameter of a cylindrical charge at which steady-state detonation (unconfined) takes place consistently is termed as the critical diameter (CD). Below CD the explosives behavior is unpredictable both in terms of initiation and performance. The lowest diameter at which ideal detonation is achieved (when max VOD is attained) is termed as IDEAL diameter (ID). It would make sense if the blasting is carried out at this diameter but may not always be possible due to other constraints.
The values of CD and ID are reported for explosive charges using standardized initiation and the charges are not confined. The values are higher for confined explosives but can again vary depending on the degree of confinement. This also can be established while experimenting to obtain comparable results (Table 2.1).
Table 2.1 Velocity of Detonation of Various Explosives

Detonation pressure and detonation velocity are two of the most important parameters for an explosive used extensively to judge its closeness to ideal detonation.
The two terms are interlinked in the form of a cause and effect situation and this relationship is given in Eq. (2.1).
2.1 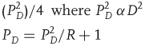
where P is density of the unreacted explosive and 4 is a calculated value. R is the ratio of specific heats of the detonation products given (i.e., 3 in most cases). There are also theoretical calculations for estimating the detonation velocity. Readers interested in studying these and also to understand the detonics of explosion can look into relevant chapters of the book by Cooper [12].
For the purposes of this book, the commercial explosives' velocity detonation is a measured value which can be easily worked out by experimental set ups ranging from the classical D'Autriche method to electronic measurements. The latter are very useful in tracing the VOD as the detonation wave progresses and are often used for measurement in a borehole during blasting.
While it is true that volumes have been spoken and written about detonation, pressure/VOD, in practice it is best used as a figure to indicate the state of the explosive in comparison to (i) ideal or theoretical maximum and (ii) figure at the start of a life cycle of the explosive.
It is also to be kept in mind that explosive performance depends not only on the detonation pressure but also on other work forces such as those generated by expanding gases formed during decomposition of the explosive ingredient(s). Several studies including underwater energy measurements estimate that out of the total energy expended by an explosive only 15–20% is partitioned as shock energy (from detonation pressure) and balance from gas/bubble energy from expanding gases.
However, there is no doubt that the shattering effect (or ability to impart movement to metal objectives is totally related to detonation pressure. Since PD is connected directly to VOD (D) it is obvious that factors affecting VOD such as density, diameter, particle size, and homogeneity of the explosive will also influence detonation pressure.
In general the effect of density has been theoretically and practically studied in pure explosives and mixtures. It has been established that PD is proportional to P2 and hence D is proportional to P. Increase of density increases the detonation velocity and consequently the detonation pressure and vice versa. The fact that an increase in bulk density beyond a certain point also brings in reduced sensitivity to initiation limits the usefulness of the above findings in practice. Behavior is contradictory for pure explosives, and those containing AN as the main component. The influence of particle size/homogeneity while understood better in pure explosives has not been so well established in mixtures based on AN. Decreasing particle size does not necessarily lead to higher sensitivity. Table 2.2 provides VOD and energies of some well-known types of commercial explosives [13].
Table 2.2 Partition of Energy
While we have previously discussed the influence of pressure developed in a detonation, we take a look now at the chemical reaction in the explosive. It is recognized that an explosive undergoing detonation releases a lot of heat (energy). Many of the injuries suffered in accidents involving explosives are burn injuries suffered due to intense heat. In practice, this huge release of heat energy goes in accelerating the chemical reaction and enabling pressure build up (spike) in a super fast time scale.
The heat of reaction also in certain cases termed as heat of detonation is the energy difference between the reactants and products. Enthalpy is the measure employed to quantify the energy in a chemical substance (Eq. (2.2)).
2.2 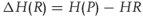
where ΔH(R) = heat of reaction,
H(P) = enthalpy of products, and
HR = enthalpy of reactants.
Although the heat of reactions can be empirically found [14], ΔH values are somewhat inconsistent as the type and quantity of products obtained depend on several parameters connected with the explosive itself and conditions under which it detonates such as initial density, degree of confinement, composition including the presence of metals, particle size, and structure. For experimental determination of product gases, qualitative and quantitative tests are preferred, but under field conditions of blasting they are not at all easy to gather.
A reasonable approximation of ΔH is obtained by following certain rules of hierarchy consistently so that products obtained are close to ideal. The rules state that
Some researchers advocate conversion of all remaining oxygen after formation of water to go toward formation of carbon dioxide. This method yields higher estimate of heat of reaction than the first one. ΔH values are well published by many sources [5, 11, 12]. Riggs has suggested a novel method of estimating the reaction products to a value near to that obtained by more sophisticated methods. Here the first use of oxygen is to form CO, and the remaining O2 is split between CO and H2 to produce CO2 and H2O. Since enthalpy values depend on product concentrations, it is assumed that calculation of heat energy released is an approximation. The detonation products emerging from the reaction zone of a detonation wave have transient existence and react with other components and form new products.
The concept arises out of the oxidation reaction, which is basis for explosive energy. The explosive energy tied up with production of heat/gaseous products depends on the oxygen balance present in the explosive. This is applicable to chemical mixtures acting as an explosive composition also.
Knowledge of oxygen balances influence on explosive properties will help in formulating an explosive with optimum performance.
Heat of reaction of an explosive reaches its maximum when it has just enough oxygen to convert all its fuel (C, H) to this higher oxidation state (CO2, H2).
Calculations of oxygen balance are well standardized as shown in Eq. (2.3). Using Eq. (2.3), the oxygen balance for ammonium nitrate is derived to be 20.
2.3 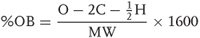
where
O = oxygen,
C = carbon,
H = hydrogen, and
MW = molecular weight.
We have seen that for maintaining oxygen balance of an explosive we may have to add fuel if it is oxygen-rich or add oxygen-giving substance (oxidant – oxidizer) if it is oxygen-deficient. In order to calculate fuel values, the same hierarchical process is used in reverse. The molecular weight of compound is divided by the oxygen deficiency using Eq. (2.4).
2.4 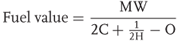
where
MW = molecular weight,
C = carbon, and
H = hydrogen.
Oxidizer value is calculated using Eq. (2.5).
2.5 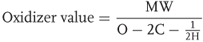
where
MW = molecular weight,
C = carbon, and
H = hydrogen.
It is to be noted that these values are not always correct as derived from the above formulas as other competing reactions like hydrogen combining with chlorine or sodium getting oxidized and giving a solid residue are not taken into account.
References
1. Konya, C.J. (1995) Blast Design, Intercontinental Development Corporation, Montville, OH.
2. Fordham, S. (1966) High Explosives and Propellants, Pergamon Press, Oxford.
3. Urbanski, T. (1964–1984) Chemistry and Technology of Explosives, vol. I–IV, Pergamon Press, Oxford.
4. Cook, M.A. (1958) The Science of High Explosives, Rheinhold Publishing Corp, New York.
5. Agrawal, J.P. (2010) High Energy Materials, Wiley-VCH Verlag GmbH, Weinheim.
6. Cook, M.A. (1974) The Science of Industrial Explosives, IRECO Chemicals, Salt Lake City, UT.
7. Johansson, C.H. and Persson, P.A. (1970) Detonics of High Explosives, Academic Press, London.
8. Bowden, F.P. and Yoffe, A.D. (1952) Initiation and Growth of Explosives in Liquids and Solids, Cambridge University Press, Cambridge.
9. Taylor, J. (1952) Detonation in Condensed Explosives, University Press, New York.
10. Zeldovich, J.B. and Kompaneets, A.S. (1960) Theory of Detonation, Academic Press, New York.
11. Riggs, R.S. Elements of Explosive Behavior, Jet Research Center, Arlington.
12. Cooper, P.W. (1996) Explosives Engineering, Wiley-VCH Verlag GmbH, Weinheim.
13. Cameron, A.R. and Torrance, A.C. (1990) Underwater evaluation of the performance of bulk commercial explosives. SEE Annual Conference, Orlando, FL.
14. Kubota, N. (2002) Thermo Chemical Aspects of Combustion, Wiley-VCH Verlag GmbH, Weinheim.
Chapter 3
Ammonium Nitrate Explosives
In this book, we deal with ammonium nitrate/fuel oil (AN/FO), slurries, and water gels, emulsions. These types of explosives are containing AN in one form or the other and in general when commercially manufactured do not contain self-explosive ingredients. It is possible that while formulating small-diameter cap-sensitive products, borderline compounds like methyl amine nitrate could be included for obtaining higher sensitivity. Bulk or large-diameter products do not include even such compounds and hence are classified as blasting agents with even less stringent rules of storage and transportation.
It is claimed that even 150 years ago, AN was known as a compound which could be used in explosives or possessed explosive properties. However nitroglycerine (NG) explosives dominated this scene till about 1950. Even during the golden period of NG explosives, AN was used as an ingredient in so-called special gelatins to provide for the reduction in the NG content and to reduce sensitivity to manufacture and handling. AN used was in crystalline form. The first commercial application of AN as major ingredient in explosive happened in 1950 when AN was mixed with carbon as fuel and used in large boreholes. Subsequently in USA the fertilizer industry expanded rapidly and AN in the form of prills became available. Initially the same type of prills were used both by the fertilizer and explosive industry, but subsequently due to the difficulty in initiation and propagation even in large boreholes search into identifying properties leading to more sensitive prills began in earnest and soon a set of specifications were developed for prills useful to the explosives industry. These are given in detail in the later part of the book but in general it was for a lower density (LD), porous structure and for thermal and physical stability, flowability. Ammonium nitrate mixed with fuel oil became a dominant product and use of NG explosives became reduced and practically no NG-based explosives was used in large-diameter blasting in USA by mid-1960s and this spread to other parts of the world subject to availability of prills. The inefficiency of AN/FO in smaller diameters and in watery holes gave rise to evolution of slurry explosives and later emulsions in a big way.
The research group under Prof. M. A. Cook in Utah, USA, did pioneering work [1] in putting slurry explosives in the forefront and subsequently water gels came on the scene for packaged and cap-sensitive explosives for all types of applications including coal mines. The difference in slurries and water gels was basically in the physical structure of the explosives. While slurry was in a fluid form, water gels were semisolid once packed. The ingredients were AN as the major component present as a supersaturated aqueous solution. Special ingredients were added to get the desired physical consistency and were sensitized in different ways [2]. While AN/FO explosives consisted of only two to three ingredients, slurries/water gels had 8–10. Certain deficiencies noticed in water gels such as their diminishing sensitivity to initiation at cold temperatures, greater tendency toward nonideal detonation, irregular behavior in watery holes with time necessitated further search and emulsion explosives came into being in many places like USA, Sweden, India, South Africa, and China. The emulsions started with the concept of AN/FO in that they used AN and fuel oil but more intimate contact between them was built up through emulsification in water-based dispersions. Emulsion explosives have become extremely sought after as they are very amenable to high-speed pumping so that boreholes could be loaded in situ very fast. Also the velocity of detonation (VOD) in small diameter and critical diameter measured in well-made emulsion explosives indicate closeness to ideal detonation not achieved in other types of AN explosives indicating a more homogeneous structure and intimate contact between particles of oxidizer (AN) and fuel through the emulsification process.
Thus currently the scenario in the commercial civil explosives is that for specialized small-diameter cap-sensitive explosives requirements, tailor-made packaged emulsion or water gel product is used. On the other hand for most large diameter blasting where large volumes are required, pumpable emulsions are preferred. Pumpable slurries are also still being used in a few locations depending on the closeness of the base plant.
The basics of maintaining close to zero oxygen balance (OB) is the first principle followed while designing good commercial explosives, whatever may be the type. This is to ensure that the explosive performs at its maximum potential and also that postdetonation fumes are least toxic.
In simple terms, the explosive consists of a mixture of oxidizer, fuel, sensitizer, and filler.
OB is calculated for any given formula by summing up of individual OB values obtained after giving due consideration to their individual quantities present in the total composition as a percentage.
The easiest way to theoretically calculate it is the case of AN/FO. AN has OB of + 0.20 and FO has a value of − 3.3 (both calculated using method discussed later). Balanced fully, the ratio of AN to FO would be 94.3/5.7. In the case of a typical water gel, it could be based on the formula
| AN 62.5% ( + 20) | + 12.5 |
| Other oxidizers 8.0% ( + 45) | +3.6 |
| Total oxygen available | + 16.1 |
| Contribution needed from fuel | − 16.1 |
In case of packaged product, there has been much debate whether the wrapper, usually polyethylene, is taking part in the reaction or not. It becomes significant only in small diameters where up to 4–5% of explosive weight could be plastic. In case of large-diameter packages, the amount of plastic would be much less − 0.5%. Formulators are well advised to keep the OB near zero without considering influence of packing material on the oxygen balance.
The design of the explosive apart from the composition has to take care the physical and performance requirements and safety within the overall cost structure allowed by market consideration.
The physical requirements are guided by the type of explosive needed (cap-sensitive or booster-sensitive), its size (diameter and weight), its packaging, and delivery mode.
In case of AN/FO, the options for design are limited as the explosive is present only as a dry mix.
The safety considerations need to meet local safety rules for storage, transport, and manufacturer's own house standards and accordingly the design of the explosive is adjusted.
Details of the design criteria for the three individual products (AN/FO, slurries, and water gels, emulsions) are given separately in chapters devoted exclusively to them.
Ever since explosives came into existence, testing them has followed. Considerable time and application of mind to device tests to assess performance and safety properties of explosives have taken place and a number of such tests are known. The tests in the earlier years [3] were oriented toward NG explosives as they were predominant during those years (up to 1960), but subsequently it was realized that the same tests could not be applied to non-NG explosives and new tests were established to encompass the newly developed AN explosives [4]. More and more importance was given to field performance of the explosives rather than their absolute properties and hence tests were designed to obtain correlation between the explosive characteristics and field performance.
A fairly comprehensive list of tests [5, 6] known and practiced are given in Table 3.1. Tests relating to strength and performance are described below:
Table 3.1 List of Tests
| Ballistic mortar test | VOD, COD, and air gap tests |
| Trauzl lead block | Plate dent test |
| Aquarium | Thermal stability |
| Double pipe test | Chemical compatibility |
| Cylinder test | Friction (torpedo) |
| Under water test | Freezing and thawing |
| Crater test | Cold temperature test |
| Hot storage test |
While discussing about the strength of an explosive, the value will have to be qualified whether it is on weight or volume basis. While two explosives can be similar in weight strength, they can differ in their volume strength because of the difference in their density. For field applications, it is the volume strength (bulk strength) that is the deciding and critical factor.
This is one of the oldest well-known tests developed a century ago and used mostly for NG-based explosives. Blasting gelatin is used as a standard [3]. A typical setup of a ballistic mortar is shown in Figure 3.1.
Figure 3.1 Ballistic mortar test. (Adapted from Mason and Aiken [4].)
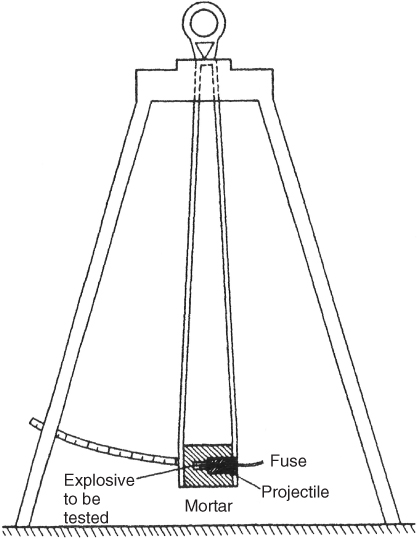
A 10-g charge of explosive is detonated inside a mortar which is suspended from a well-anchored structure and is able to swing freely. When the charge explodes, the recoil measured in terms of maximum deflection is noted and compared with standard deflection obtained by use of a 10-g charge of blasting gelatin. Very reproducible results were obtained for NG-based explosives and the strength of these explosives was very often quoted as percentage of blasting gelatine (BG). However the test did not give reproducibility with non-NG explosives especially with AN/FO where in many instances the explosive did not fully detonate and traces were left behind. Even cap-sensitive emulsions and water gels showed variable results though reproducibility was better than that with AN/FO.
Factors affecting the test results and leading to variability were loading density, sensitivity, speed of the decomposition reaction, and stemming variability due to personal factor.
Thus currently this test is not practiced on a routine basis with AN-based explosives and blasting agents. Several studies to correlate weight strength values obtained by this method with bubble energy (gas energy) and shock energy did not yield any meaningful relationship.
This is another classical test developed in the early twentieth century and used for NG explosives and other molecular explosives. The test was used to measure and compare strengths while developing different formulations. Later it was also used as a type test to keep track of the product being manufactured and in storage.
Herein again it was found that the method did not yield reproducible results for non-cap-sensitive products, but for cap-sensitive explosives such as aluminized water gels the results obtained were good [7] and the values could be correlated with calculated energy and total underwater energy.
The test is simple and consists of detonating with a number 6 detonator, ten grams of explosive in a cast lead block with a 25-mm-diameter hole drilled in the center to a depth half way from the bottom (200 mm). The charge placed at the bottom of the drilled hole is surrounded on three sides by lead block walls of sufficient thickness to prevent a blow hole. The remaining side (top) is stemmed with loose sand. For standardization and reproducibility, lead blocks cast from the same mold, composition, and dimensions are used. Also same type, quality, and quantity of fine sand for stemming and identical number 6 ordinary detonators are used. The explosive is contained in an aluminum foil of the same dimension every time. Once the charge explodes, the gases released expand the lead block and enlarge the volume inside (Figure 3.2).
Figure 3.2 Lead block expansion test (Trauzel test).
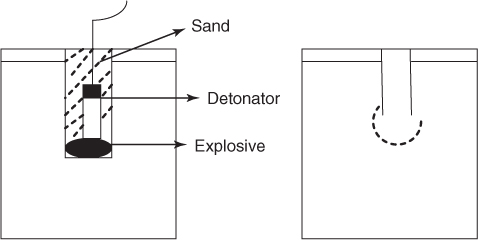
The increase in volume of the hole in the lead block before and after detonation measured accurately by filling up to the brim with H2O from a burette will give the volume increase only due to the explosive and is a measure of its weight strength. To remove the influence of the strength of the detonator used in the test, the volume of expansion in a lead block of a standard detonator is measured and used as a correction factor when obtaining the work done by the explosive.
Here again either BG or a standard NG explosive such as a special gelatin 80% is used for comparison.
While the ballistic mortar and lead block tests measure the work done by expanding gases behind the detonation wave, there are a few tests that measure the shock energy due to detonation wave as well as reaction velocity itself.
It is a very common quoted figure to indicate the speed at which the reaction moves once the explosive is initiated. It also gives the condition of the explosive when its VOD is measured and compared with the maximum VOD attained or the ideal VOD achievable by that explosive. If the measured VOD is much lower than the latter, it means that the explosive is detonating at a reduced performance level and will not give the desired results in the borehole although confinement generally improves the VOD of most explosives by 10–15%. The diameter also has an influence on the VOD, and greater the diameter more is the VOD till a constant. VOD value is reached for that particular explosive (VODmax). The VOD measurement as standardized is conducted in the open without confinement and in the diameter for which VOD is required to be measured. The initiation is also standardized. For cap-sensitive explosives, the number 6 detonator is used and for LD non-cap-sensitive explosives pentaerythritol tetranitrate/trinitrotoluene (PETN/TNT) booster is used, the latter being initiated either by a detonator or by a detonating fuse. The diameter of the booster should be as close as possible to the diameter of the cartridge being tested and the weight used is about 10% of the explosive weight.
The actual most used method for experimental determination of the VOD known as D'Autriche method consists of the setup as shown in Figure 3.3.
Figure 3.3 D'Autriche method for VOD determination.
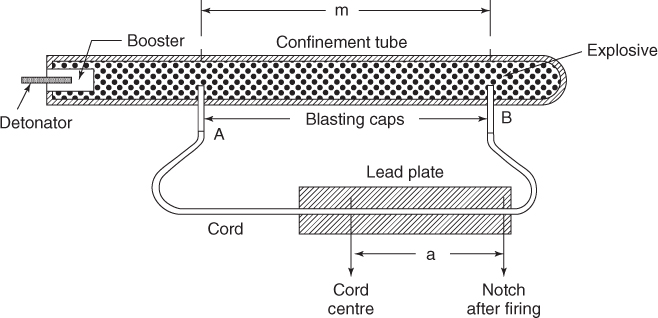
As the reaction proceeds after initiation, it sets off the two arms (probes) of the detonating fuse embedded at a fixed distance in the cartridge. The detonating fuse after getting initiated has two waves traveling in the opposite direction. They meet at a point and the collision is marked on a lead or aluminum plate. The distance of the mark on the plate from the midpoint of the DF used is a measure of the VOD of the explosive. A standardized value for the DF is used after it is calibrated and used in the equation:
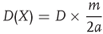
where:
D(X) = the VOD of the explosive under test in m/s,
D = VOD of the calibrated detonating fuse used in the test in meters per second,
a = distance between the mark on the witness plate and center of the detonating fuse in centimeter, and
m = length between the probes in cm.
Over the years, the DF probe has been replaced by electronic devices based on fiber optics so the continuous measurement of VOD over the length of the cartridge can be measured. Electronic methods when they function well give very accurate results, but most routine testing is done by the classical method. However if the VOD needs to be measured in a borehole, electronic methods are the only ones which can be used.
Some precautions needed to be observed while measuring VOD by D'Autriche method in that care should be taken in
It also needs to be understood that while VOD is a good measure of the condition of the explosive, it is much less useful in predicting the behavior of the explosive in the borehole.