

Table of Contents
Preface
Acknowledgements
Contributors
Disclaimer
Section 1: Introduction
CHAPTER ONE: The Role of the Veterinary Technician in Exotic Animal Medicine
Section 2: Avian
CHAPTER TWO: Psittacines and Passerines
INTRODUCTION
ANATOMY AND PHYSIOLOGY
COMPARATIVE CLINICAL PATHOLOGY
NUTRITION
HISTORY, RESTRAINT, AND PHYSICAL EXAMINATION
COMMON DISEASES
RADIOLOGY
ANESTHESIA AND ANALGESIA
SURGERY
PARASITOLOGY
GENDER DETERMINATION
GROOMING
EMERGENCY AND CRITICAL CARE
TECHNIQUES
ADMINISTRATION OF MEDICATIONS
DIAGNOSTIC SAMPLING
WOUND CARE AND BANDAGING
EUTHANASIA
REFERENCES
CHAPTER THREE: Psittacine Behavior, Husbandry, and Enrichment
INTRODUCTION
BEHAVIOR OF COMMON PET PSITTACINE SPECIES
HUSBANDRY
NORMAL BEHAVIOR OF PSITTACINE BIRD SPECIES
ABNORMAL BEHAVIOR
ADDITIONAL READING
CHAPTER FOUR: Aviary Design and Management
INTRODUCTION
AVICULTURE
QUARANTINE
EXAMINATIONS AND DIAGNOSTIC TESTING: NEONATES, JUVENILES, BREEDERS, AND NEW ACQUISITIONS
DISINFECTION AND DISEASE PREVENTION
PEDIATRICS FOR THE AVICULTURIST
CONCLUSION
ADDITIONAL READING
CHAPTER FIVE: Sex Differentiation and Reproduction
INTRODUCTION
SEX DIFFERENTIATION
REPRODUCTION
REPRODUCTIVE MEDICINE AND SURGERY
ADDITIONAL READING
Section 3: Reptiles
CHAPTER SIX: Lizards
INTRODUCTION
ANATOMY AND PHYSIOLOGY
HUSBANDRY
QUARANTINE
NUTRITION
COMMON DISORDERS
BEHAVIOR
TOXICITY AND MISCELLANEOUS NUTRITIONAL DISORDERS
ZOONOSES
HISTORY, RESTRAINT, AND PHYSICAL EXAM
RADIOLOGY
ANESTHESIA AND SURGERY
PARASITOLOGY
EMERGENCIES
TECHNIQUES
EUTHANASIA
REFERENCES
CHAPTER SEVEN: Snakes
INTRODUCTION
CAPTIVE-BRED VERSUS WILD-CAUGHT
BEHAVIOR
ANATOMY AND PHYSIOLOGY
REPRODUCTIVE BIOLOGY AND HUSBANDRY
EGG ANATOMY
EGG INCUBATION AND MANAGEMENT
HOUSING
QUARANTINE
NUTRITION
TRANSPORTATION
DISEASES AND CLINICAL CONDITIONS
TAKING A HISTORY
PREPARING FOR THE PHYSICAL EXAM
RESTRAINT
THE PHYSICAL EXAM
RADIOLOGY
ANESTHESIA
SURGERY
PARASITOLOGY
COMMON PARASITES OF SNAKES
EMERGENCY AND CRITICAL CARE
EMERGENCY CONDITIONS
CRITICAL CARE MONITORING
SEX DETERMININATION
CLINICAL TECHNIQUES
VENOMOUS SNAKES
EUTHANASIA
BEING A RESPONSIBLE SNAKE OWNER
REFERENCES
CHAPTER EIGHT: Chelonians
INTRODUCTION
ANATOMY AND PHYSIOLOGY
HUSBANDRY AND NUTRITION
COMMON DISEASES
ZOONOSES
OBTAINING A HISTORY AND PERFORMING A PHYSICAL EXAMINATION
RESTRAINT
RADIOLOGY
ANESTHESIA
PARASITOLOGY
EMERGENCY AND CRITICAL CARE
CLINICAL TECHNIQUES
ADMINISTRATION OF MEDICATIONS
EUTHANASIA
REFERENCES
CHAPTER NINE: Herpetoculture and Reproduction
INTRODUCTION
CAPTIVE-BRED VERSUS WILD-CAUGHT
QUARANTINE
MANAGING LARGE COLLECTIONS
MANAGING LARGE COLLECTIONS OF DANGEROUS SPECIES
METHODS OF SEX DETERMINATION
REPRODUCTIVE BEHAVIOR
FOLLICLE AND EGG DEVELOPMENT
CLUTCH DYNAMICS
OVIPAROUS, OVOVIVIPAROUS, OR VIVIPAROUS
EGG INCUBATION VERSUS MATERNAL INCUBATION
EGG INCUBATION METHODS
DIAGNOSING EGG PROBLEMS
CARING FOR THE NEWBORN
REFERENCES
Section 4: Amphibians
CHAPTER TEN: Amphibians
INTRODUCTION, TAXONOMY, AND NATURAL HISTORY
ANATOMY AND PHYSIOLOGY
HUSBANDRY
ENCLOSURE DESIGN
AMPHIBIAN-ENVIRONMENT INTERACTION
AMPHIBIAN-AMPHIBIAN INTERACTION
QUARANTINE
NUTRITION
FOOD ITEMS
COMMON DISORDERS
LARVAL AMPHIBIANS
HISTORY AND PHYSICAL EXAM
RADIOLOGY
ANESTHESIA AND SURGERY
TECHNIQUES
EUTHANASIA
REFERENCES
Section 5: Mammals
CHAPTER ELEVEN: Ferrets
INTRODUCTION
ANATOMY
BEHAVIOR
HUSBANDRY
NUTRITION
COMMON AND ZOONOTIC DISEASES
HISTORY AND PHYSICAL EXAMINATION
PREVENTIVE MEDICINE
RESTRAINT
RADIOLOGY AND ULTRASOUND
ANESTHESIA AND SURGERY
PARASITOLOGY
URINALYSIS
EMERGENCY AND CRITICAL CARE
SEX DETERMINATION
TECHNIQUES
REFERENCES
CHAPTER TWELVE: Rabbits
INTRODUCTION
BEHAVIOR
ANATOMY AND PHYSIOLOGY
BIOLOGIC AND REPRODUCTIVE DATA
HUSBANDRY
NUTRITION
COMMON AND ZOONOTIC DISEASES
TAKING A HISTORY
PHYSICAL EXAMINATION AND PREVENTIVE MEDICINE
RESTRAINT
RADIOLOGY
ANESTHESIA
COMMON SURGICAL PROCEDURES
PARASITOLOGY
URINALYSIS
CLINICAL TECHNIQUES
SEX DETERMINATION
EMERGENCY AND CRITICAL CARE
EUTHANASIA
REFERENCES
CHAPTER THIRTEEN: Mice, Rats, Gerbils, and Hamsters
INTRODUCTION
ANATOMY AND PHYSIOLOGY
BIOLOGIC AND REPRODUCTIVE DATA
HUSBANDRY
NUTRITION
COMMON PARASITES, DISEASES, AND ZOONOSES
BEHAVIOR
HISTORY AND PHYSICAL EXAMINATION
RESTRAINT AND HANDLING
RADIOLOGY
SURGERY AND ANESTHESIA
BANDAGING AND WOUND CARE
EMERGENCY AND CRITICAL CARE
SEX DETERMINATION
TECHNIQUES
EUTHANASIA
ADDITIONAL READING
CHAPTER FOURTEEN: Chinchillas
TAXONOMY/COMMON SPECIES SEEN IN PRACTICE
ANATOMY AND PHYSIOLOGY
REPRODUCTION
HUSBANDRY
NUTRITION
COMMON AND ZOONOTIC DISEASES
BEHAVIOR
TAKING THE HISTORY AND PERFORMING THE PHYSICAL EXAM
HANDLING AND RESTRAINT
RADIOLOGY
ANESTHESIA AND SURGERY
PARASITOLOGY
URINALYSIS
EMERGENCY AND CRITICAL CARE
SEX DETERMINATION
TECHNIQUES
EUTHANASIA
REFERENCES
CHAPTER FIFTEEN: Guinea Pigs
COMMON TYPES
BEHAVIOR
ANATOMY AND PHYSIOLOGY
BIOLOGIC AND REPRODUCTIVE DATA
HUSBANDRY
NUTRITION
COMMON AND ZOONOTIC DISEASES
HISTORY AND PHYSICAL EXAMINATION
RESTRAINT
RADIOLOGY
ANESTHESIA AND SURGERY
PARASITOLOGY
URINALYSIS
EMERGENCY AND CRITICAL CARE
SEX DETERMINATION
TECHNIQUES
EUTHANASIA
REFERENCES
CHAPTER SIXTEEN: Hedgehogs
TAXOMONY, ANATOMY, AND PHYSIOLOGY
BIOLOGIC AND REPRODUCTIVE DATA
BEHAVIOR
HUSBANDRY
NUTRITION
COMMON DISEASES
OBTAINING A HISTORY AND PHYSICAL EXAMINATION
RADIOLOGY
ANESTHESIA AND SURGERY
URINALYSIS
PARASITOLOGY
EMERGENCY AND CRITICAL CARE
SEX DETERMINATION
TECHNIQUES
REFERENCES
CHAPTER SEVENTEEN: Skunks
INTRODUCTION
ANATOMY AND PHYSIOLOGY
HUSBANDRY AND NUTRITION
COMMON HEALTH PROBLEMS
AMYLOIDOSIS
ZOONOTIC DISEASES
PHYSICAL EXAMINATION
VACCINATIONS
RESTRAINT
RADIOLOGY
ANESTHESIA
PARASITOLOGY
CLINICAL TECHNIQUES
ADMINISTRATION OF MEDICATIONS
REFERENCES
CHAPTER EIGHTEEN: Sugar Gliders
INTRODUCTION
ANATOMY
HUSBANDRY AND NUTRITION
COMMON DISEASES
PHYSICAL EXAMINATION
RESTRAINT
RADIOLOGY
ANESTHESIA
PARASITOLOGY
CLINICAL TECHNIQUES
REFERENCES
CHAPTER NINETEEN: Prairie Dogs
INTRODUCTION
ANATOMY AND PHYSIOLOGY
HUSBANDRY AND NUTRITION
COMMON AND ZOONOTIC DISEASES
RESTRAINT
RADIOLOGY
ANESTHESIA
CLINICAL TECHNIQUES
REFERENCES
Section 6: Wildlife Rehabilitation
CHAPTER TWENTY: The Role of the Veterinary Technician in Wildlife Rehabilitation
INTRODUCTION
GETTING STARTED
REHABILITATING WILDLIFE IN A SMALL ANIMAL VETERINARY HOSPITAL
CLINIC PROTOCOLS
INTAKE PROCEDURES
ETHICAL CONSIDERATIONS AND REDUCING STRESS IN CAPTIVE WILDLIFE
INITIAL EXAM
CHOOSING TREATMENT ROUTES
RELEASE CRITERIA VERSUS EUTHANASIA
IMPRINTING AND TAMING
TRANSPORTING WILDLIFE
RAPTOR CARE
ALTRICIAL ORPHAN SONGBIRDS
CARING FOR ADULT PASSERINE (SONG) BIRDS
PRECOCIAL BIRD BASIC CARE
ADULT PRECOCIAL BIRDS (INCLUDING WATERFOWL AND WADING BIRDS)
GENERAL ORPHAN MAMMAL CARE
SPECIES CARE SHEETS
ACKNOWLEDGMENTS
REFERENCES
Section 7: Hematology
CHAPTER TWENTY-ONE: Avian and Reptile Hematology
INTRODUCTION
BLOOD COLLECTION
BLOOD SMEAR AND ASSESSMENT
LEUKOCYTES
ERYTHROCYTES
THROMBOCYTES
REFERENCES
APPENDIX ONE: Supplies Necessary for an Exotic Practice
UNITED STATES MIGRATORY BIRD PERMIT OFFICES
APPENDIX TWO: Wildlife Admission/Exam/Care Forms
APPENDIX THREE: Handling and Restraint of Wildlife Species
INTRODUCTION
CONSIDERATIONS
PREPARATION
TOOLS OF RESTRAINT
SPECIFIC TECHNIQUES BY SPECIES
CONCLUSION
REFERENCE
APPENDIX FOUR: Tail Wrapping
APPENDIX FIVE: Guide to Identification of Hatchling and Nestling Songbirds
APPENDIX SIX: Average Body Weights of Selected North American Songbirds
APPENDIX SEVEN: Species Care Sheets
RACCOONS
FLYING SQUIRRELS
OPOSSUMS
GREY SQUIRRELS
COTTONTAIL RABBITS
APPENDIX EIGHT: Biological Data of Selected North American Wild Mammals
APPENDIX NINE: Glossary of Medical Conditions and Treatments
APPENDIX TEN: Wildlife Product Sources
APPENDIX ELEVEN: Additional Resources
NATURAL HISTORY: GENERAL
BIRDS
RODENTS
LAGOMORPHS
OPOSSUMS
CANIDS
MUSTELIDS
PROCYONS
WILDLIFE REHABILITATION ORGANIZATIONS
REHABILITATION BOOKS, MANUALS, AND CDs
WEBSITES USEFUL TO REHABILITATORS
INTERNET LISTSERVE FOR PROFESSIONAL WILDLIFE REHABILITATORS
APPENDIX TWELVE: Supplies Necessary for an Exotic Practice
Index

First edition first published 2003
Second edition first published 2010
© 2010 Blackwell Publishing
Blackwell Publishing was acquired by John Wiley & Sons in February 2007. Blackwell’s publishing program has been merged with Wiley’s global Scientific, Technical, and Medical business to form Wiley-Blackwell.
Editorial Office
2121 State Avenue, Ames, Iowa 50014-8300, USA
For details of our global editorial offices, for customer services, and for information about how to apply for permission to reuse the copyright material in this book, please see our website at www.wiley.com/wiley-blackwell.
Authorization to photocopy items for internal or personal use, or the internal or personal use of specific clients, is granted by Blackwell Publishing, provided that the base fee is paid directly to the Copyright Clearance Center, 222 Rosewood Drive, Danvers, MA 01923. For those organizations that have been granted a photocopy license by CCC, a separate system of payments has been arranged. The fee codes for users of the Transactional Reporting Service are ISBN-13: 978-0-8138-2206-8/2010.
Designations used by companies to distinguish their products are often claimed as trademarks. All brand names and product names used in this book are trade names, service marks, trademarks or registered trademarks of their respective owners. The publisher is not associated with any product or vendor mentioned in this book. This publication is designed to provide accurate and authoritative information in regard to the subject matter covered. It is sold on the understanding that the publisher is not engaged in rendering professional services. If professional advice or other expert assistance is required, the services of a competent professional should be sought.
Library of Congress Cataloging-in-Publication Data
Exotic animal medicine for the veterinary technician / edited by Bonnie Ballard and Ryan Cheek. – 2nd ed.
p. ; cm.
Includes bibliographical references and index.
ISBN-13: 978-0-8138-2206-8 (alk. paper)
ISBN-10: 0-8138-2206-8 (alk. paper)
1. Exotic animals–Diseases. 2. Wildlife diseases. 3. Pet medicine. 4. Veterinary nursing. I. Ballard, Bonnie M. II. Cheek, Ryan.
[DNLM: 1. Animal Diseases. 2. Animals, Wild. 3. Animal Technicians. 4. Veterinary Medicine–methods. SF 997.5.E95 E96 2010]
SF997.5.E95C44 2010
636.089′073–dc22
2009035848
A catalog record for this book is available from the U.S. Library of Congress.
Disclaimer
The contents of this work are intended to further general scientific research, understanding, and discussion only and are not intended and should not be relied upon as recommending or promoting a specific method, diagnosis, or treatment by practitioners for any particular patient. The publisher and the author make no representations or warranties with respect to the accuracy or completeness of the contents of this work and specifically disclaim all warranties, including without limitation any implied warranties of fitness for a particular purpose. In view of ongoing research, equipment modifications, changes in governmental regulations, and the constant flow of information relating to the use of medicines, equipment, and devices, the reader is urged to review and evaluate the information provided in the package insert or instructions for each medicine, equipment, or device for, among other things, any changes in the instructions or indication of usage and for added warnings and precautions. Readers should consult with a specialist where appropriate. The fact that an organization or Website is referred to in this work as a citation and/or a potential source of further information does not mean that the author or the publisher endorses the information the organization or Website may provide or recommendations it may make. Further, readers should be aware that Internet Websites listed in this work may have changed or disappeared between when this work was written and when it is read. No warranty may be created or extended by any promotional statements for this work. Neither the publisher nor the author shall be liable for any damages arising herefrom.
1 2010
Preface
The second edition was written to provide the veterinary technician with important information about a variety of species commonly seen in exotic practice, reflecting changes in this branch of medicine that have occurred since the first edition. This text is beneficial to the technician who would like to work with these animals but may have graduated years ago, before this area of medicine was popular. This text is also helpful to the technician who works for a veterinarian who would like to add exotic species to his/her practice. While it was not written for veterinarians, they may find it beneficial as well.
With the help of this book, the technician will know what questions to ask to obtain an adequate history, be able to educate the client about husbandry and nutrition, be able to safely handle and restrain common species, and be able to perform necessary procedures when needed. Because the field of exotic animal medicine is a dynamic one, new knowledge is constantly emerging about many of the species kept as pets, and new information can, in some cases, contradict what was thought to be true before. For many species, exotic animal medicine can be said to be in its infancy. We realize that for some of the species featured in this book, the information presented may need to be modified in the future. Further, because what we know about exotic animal medicine is forever changing and much has not been scientifically proven, it is common to find contradicting information from one reputable source to the next. This can create frustration but also provide the challenge of working in a cutting edge area of medicine. This is the major reason why it is paramount to attend continuing education in this area of medicine. Veterinary technicians working in exotic medicine must engage in lifelong learning to be up to date on the latest information.
New contributors as well as new chapters have been added to this edition. Although some of the contributors have provided drug dosages and formularies, we do not take responsibility for what this information. We also realize that while technicians do not make decisions about what drugs to use in any animal, they are required to be familiar with different pharmaceuticals, know where to find a dosage, and know how to calculate it.
This book was written with the assumption that the technician already is educated in topics such as anatomy, physiology, medical terminology, pathology, and pharmacology. We present only what is unique to the species featured here.
We hope this book proves to be beneficial to all technicians interested in exotic animal medicine.
Acknowledgements
I would like to thank all of our new contributors who gave their time to provide additional information to enhance this edition. Appreciation also goes to the original contributors who took time to update their original chapters. I would like to thank my husband Brian Kershaw for continually being supportive and understanding when I had to take my precious little free time to work on the book!
Bonnie Ballard
This second edition has seen many changes. I would like to thank my family and friends for the support they have given me throughout this entire process. I would also like to acknowledge the many technicians working in the field of exotic animal medicine. This is an ever-changing and evolving field that requires dedication and patience. Your commitment to this field is truly inspiring.
Ryan Cheek
Bonnie Ballard, DVM, has worked in veterinary medicine since 1974, starting as a veterinary assistant, becoming a technician in 1979, and earning a DVM in 1994. In 1997, she started the veterinary technology program at Gwinnett Technical College. The program has been AVMA accredited since 2000. Dr. Ballard currently is the program’s director and one of two full time faculty members. She has won numerous teaching awards and has received many accolades for the program. She also practices small animal and exotic medicine at Winder Animal Hospital in Winder, Georgia.
Denise I. Bounous, DVM, PhD, Diplomate ACVP, was a professor of clinical pathology at the University of Georgia College of Veterinary Medicine before her move into the pharmaceutical industry. Her academic interests included avian and reptilian clinical pathology, and her research was in avian immunomodulation.
Ryan Cheek graduated from Gwinnett Technical College in 1999 with an Associate’s Degree in Applied Technology in veterinary technology, where he focused his studies on exotic animal medicine. He worked at Zoo Atlanta and then at a small animal/exotic animal practice for four years. He has worked in emergency and critical care for the past eleven years. He completed his Veterinary Technician Specialist in Emergency and Critical Care in 2005 and his Bachelor’s of Applied Science Degree in veterinary technology from St. Petersburg College in 2007. He has taught full time at Gwinnett Technical College since 2007; he teaches many subjects including exotic, wildlife, zoo, and laboratory animal medicine.
Maria M. Crane, DVM, received her MS in exercise science from Georgia State University and her DVM from the University of Georgia in 1994. She worked as a veterinarian at Zoo Atlanta, providing clinical and surgical care, and later as vice president of animal health, managing veterinary and nutritional services. She has also practiced small and exotic animal medicine.
Lillian Gerhardt, LVT, graduated from the State University of New York. She has been a technician at the University of Tennessee College of Veterinary Medicine in the Avian and Zoological Medicine Service for eighteen years. She has presented seminars at the Avian Veterinarian Annual Conferences several times. She has always had a special interest in birds and has shared the last twenty-three years of her life with a sulphur crested cockatoo named Sugar.
Cheryl B. Greenacre, DVM, Diplomate ABVP-Avian, graduated from the University of Georgia College of Veterinary Medicine in 1991 and taught avian and exotic animal medicine at UGA for ten years and at the University of Tennessee College of Veterinary Medicine for nine years. Dr. Greenacre is the immediate past chair for the UT Institutional Animal Care and Use Committee, and is currently a professor at the University of Tennessee. She divides her work time between teaching veterinary students and residents, providing clients and referring veterinarians with service, and studying thyroid testing in birds and pain relief in reptiles.
Tarah Hadley, DVM, Diplomate ABVP-Avian, is a graduate of Dartmouth College and Tufts University, where she received her DVM. She completed an internship in small animal medicine and surgery at Rowley Memorial Animal Hospital in Massachusetts and a residency in avian medicine and surgery at the University of Tennessee. During her residency, Dr. Hadley was also trained in exotic animal and zoological medicine. She currently serves as director of the Atlanta Hospital for Birds and Exotics and is a member of the veterinary staff at Zoo Atlanta.
Melanie Haire, VMT, received an AS degree in veterinary technology from Wilson College in 1987 and worked for six years in an Atlanta small animal clinic following graduation. She has spent the last fifteen years on the veterinary staff at Zoo Atlanta, where she is the senior veterinary technician and hospital manager. She is federally licensed to rehabilitate migratory bird species and raptors and has a state permit to rehabilitate wild mammals, including rabies vector species. She volunteers at the local wildlife rehabilitation center, Atlanta Wild Animal Rescue Effort (AWARE), where she is also a board member.
Anne E. Hudson, LVT, LAT, graduated from Blue Ridge Community College with an AAS in veterinary technology and received AALAS certification. She has worked as a biological laboratory technician for the Department of Defense’s Clinical Investigation and Research Department. She teaches veterinary assisting to high school students interested in pursuing careers in the veterinary field.
Michael J. Huerkamp, DVM, Diplomate ACLAM, earned his DVM from The Ohio State University and did postdoctoral training in the specialty area of laboratory animal medicine at the University of Michigan. He is a professor of pathology and laboratory medicine in the Emory University School of Medicine, where he also serves as director of the Division of Animal Resources. Dr. Huerkamp has twenty-two years of experience in the medical care and management of laboratory animals, including rabbits.
Michael Duffy Jones, DVM, received a BS from Notre Dame and DVM from Tufts University. He completed an internship at Georgia Veterinary Specialists. He worked for five years at Bells Ferry Animal Hospital before opening his own practice, Peachtree Hills Animal Hospital, in Atlanta in 2005. He has a particular interest in the use of ultrasound as a diagnostic tool, which he uses regularly in his practice and which he teaches to other veterinarians.
Vanessa Lee, DVM, obtained her veterinary degree from the University of Georgia in 2005. She was an associate veterinarian in a small animal and exotic companion animal private practice for two years and is currently in a laboratory animal medicine residency at Emory University in Atlanta, Georgia.
Trevor Lyon, RVT, graduated from Maple Woods Community College with an AA in veterinary technology. Focusing on internal medicine, he has lectured at several veterinary conferences around the country. He is a technician supervisor and co-owner of Bells Ferry Veterinary Hospital. He has worked with wildlife rehabilitation programs and participated in programs to educate the public about wildlife.
David Martinez-Jimenez, DVM, was born in Spain, where he completed his veterinary degree in 2002. After graduation, he performed several externships in exotic pet, zoo, and wildlife medicine. In 2004, he completed a Master’s Degree in Wild Animal Health at the Royal Veterinary College and Institute of Zoology of London. He then moved to the USA, where he completed an internship in exotic, zoo, and wildlife medicine at the University of Georgia College of Veterinary Medicine. Dr. Martinez-Jimenez is currently practicing in zoo, wildlife, and exotic medicine
Julie Mays, LVT, graduated from Snead State Community College with an AA in veterinary technology. She has long had an interest in exotic animal medicine and surgery.
James R. McClearen, DVM, graduated from the University of Georgia with a BS in agriculture. He received his DVM from the University of Georgia College of Veterinary Medicine, where he worked for several years in raptor rehabilitation. He sold Bells Ferry Veterinary Hospital, his small animal and exotic pet practice, in 2007, although he still works there. He is active in the Georgia Veterinary Medical Association and served as its president in 2008.
Deborah Mook, DVM, Diplomate ACLAM, received her DVM from the University of Wisconsin-Madison in 1998 and became board-certified in laboratory animal medicine in 2004. She worked with pet rabbits in the clinical setting and rabbits as research models in the medical school setting. Her primary expertise lies in the field of laboratory animal medicine, with a focus on murine infectious disease.
Shannon Goldsmith, CAT, operates a reptile and amphibian rescue program and is active in the community, providing educational programs related to these wonderful creatures.
Samuel Rivera, DVM, Diplomate ABVP-Avian, graduated from Kansas State University College of Veterinary Medicine. He later received a Masters of Science in veterinary pathobiology. After practicing in an avian and exotic practice for several years, he now serves as an associate veterinarian at Zoo Atlanta.
April Romagnano, PhD, DVM, Diplomate ABVP, obtained her PhD from the Universitæ de Montræal in 1987, and a DVM from the University of Florida in 1992. She completed an internship in wildlife/small animal medicine at the University of Florida in 1993, a residency in non-domestic avian medicine at North Carolina State University in 1995, and a post doctoral appointment in BCL2 transgenic mice at the Howard Hughes Medical Institute Research Lab at Washington University in St. Louis, Missouri, in 1988. In 2001 she opened an animal clinic and serves as the avian specialist there. She also serves as the full-time director of animal resources at Scripps Florida, a consultant veterinarian for Lion Country Safari in Loxahatchee, Florida, and a courtesy clinical assistant professor at the College of Veterinary Medicine at the University of Florida.
Douglas K. Taylor, DVM, MS, Diplomate ACLAM, received his Veterinary Degree from Michigan State University in 1995 and practiced small animal medicine for five years afterward. He received his specialty training in laboratory animal medicine at the University of Michigan, where he also earned his MS. He is currently the director of surgery and anesthesia services and the assistant director of the residency training program at Emory University in Atlanta, Georgia.
Brad Wilson, DVM, is a veterinarian and partner in two private practice veterinary clinics in north Atlanta. He received his BS in zoology and his DVM from the University of Georgia. He is the consulting veterinarian for the largest wholesale importer and distributor of fish, reptiles, amphibians, pocket pets, ferrets, and birds in north Georgia as well as for the Atlanta Botanical Garden, which has an extensive collection of dendrobatid and Central and South American hylid frogs. He has personally maintained and captively bred many species of snakes and frogs.
Disclaimer
Because exotic animal dosages are based largely on empirical data and not researched facts, the editors and contributors make no guarantees regarding the results obtained from dosages used in this textbook.
Welcome to the world of exotic animal medicine! For those who practice it, it is the variety that provides the spice to veterinary life. In a practice that sees exotics, it is not uncommon to see a dog for vaccines, a diabetic cat, an iguana with metabolic bone disease, a ferret for a physical examination, a rabbit with hair loss, and a feather-picking cockatoo all in one day. The challenge for those in this field lies in the vast differences in the species seen (Figure 1.1).
In the world of veterinary medicine, an exotic animal is any animal that isn’t a dog, cat, horse, or cow. Exotic animals include wildlife species, animals commonly used in research that are kept as pets, and animals native to various regions of the world such as South America, Australia, and Africa. Owners of “pocket pets” such as mice, rats, gerbils, and hamsters commonly seek veterinary care for their pets.
There are several scenarios in which a technician faced with exotics may find this book helpful. For instance, a technician might take a job in a practice that sees exotics but she knows little about them because she graduated before exotics became popular pets. Another technician may work for a veterinarian who wants to add exotics to the practice but doesn’t have hands-on experience with them. Alternatively, a technician who finds employment at a zoological park or works with a wildlife rehabilitator may want to brush up on current ideas about exotics. While this book does not cover zoo species specifically, knowledge of exotic animals, their treatment, and their care is desirable in the zoo environment.
It is essential that a technician who works for a veterinarian who would like to add exotics to the practice help the veterinarian understand how the practice will need to change to accommodate these species. One must accept the fact that a fifteen- or twenty-minute appointment will not suffice. In many cases appointments of thirty minutes or longer are required. Because husbandry and nutrition are typically the two most common causes of illness in exotics, a thorough history in these areas is essential. Furthermore, more time may be required to perform a physical examination due to the delicate nature of some of the species. In many cases it is necessary to allow adequate time to educate the owner about how to keep his/her pet healthy.
The front office staff must be knowledgeable and interested in exotic pets because they will be the first people the pet owner sees in the office. The worst thing that can happen for a snake owner, for example, is to step up to the front desk and see the receptionist recoil in horror. Not only is this behavior unprofessional, but it also calls into question the knowledge of the doctors. Likewise, if a receptionist does not know the difference between a macaw and a cockatoo, it may give the impression that the clinic doesn’t see many birds.
Housing is another consideration in the decision to treat exotic pets. Because many pocket pets are prey animals, their housing in relation to that of dogs and cats must be considered. For example, a rabbit should not be caged where a cat patient can watch it. This alone can create undue added stress for a rabbit patient, which is already stressed by being in the hospital environment. An exotic pet should not have to add the fear of being eaten to its worries during a hospital stay.
Figure 1.1. A technician drawing blood from a skunk. (Photo courtesy of Ryan Cheek.)

While the average animal hospital has most of the necessary equipment needed to treat exotics, some items will need to be purchased. For example, a gram scale is required to weigh many of the very small patients. Microtainer blood collection tubes are also essential. A list of equipment that is useful in exotic practices appears in Appendix 12.
The technician’s role in exotic animal medicine is the same as it is in small or large animal medicine. One of the most important roles is that of a meticulous history taker. As each chapter illustrates, a simple history will not do. Detailed questions must be asked about how and where the pet was acquired. Wild-caught species can have different health problems than those raised in captivity. How the pet is housed is vitally important, and this means not only asking what it is housed in but the cage size, construction, substrate used, and where it is kept in the house. If the animal is not brought in the cage it is housed in, the technician, after gathering the history, should be able to create a mental picture of what the cage at home looks like.
The same is true for gathering adequate information about the pet’s diet. It is not good enough to ask what is fed, because that may not be what is consumed. For example, an owner may report that his Amazon parrot’s daily diet is made up of fruits, vegetables, and seeds. When asked how much of each is consumed each day, the answer may be mostly seeds, which is an inadequate diet.
In many cases, owners of exotics may have been misinformed about their pets’ care by the pet shops where the pets were purchased. Although some pet shop employees are knowledgeable, many simply do not know the correct information about the species they sell. In addition, an owner may have read information from a less than reputable source. The veterinary technician should be able to give owners the correct information about husbandry and nutrition without chastising them for their mistakes. Many owners honestly may not know that what they were doing was wrong. They may have obtained books that are not written by reputable sources or found information on the Internet that is inaccurate. Clients value information about how to keep their pets healthy, and their veterinary clinic should be the source of that information.
The technician can also provide valuable information about what type of exotic pet a client should buy. For example, an iguana is considered to be a difficult reptile to keep because its housing and nutrition requirements are demanding. A bearded dragon may be a better choice. A parakeet may be a better choice than a macaw for a first-time bird owner, because macaws can be noisy and messy. The topic of conservation of species is important here as well. New exotic pet owners should be encouraged to acquire captive-raised species rather than wild-caught if possible. In many exotic species, the numbers in the wild are diminishing. This is especially true of many avian species. Most exotic species that are desirable as pets can be obtained from captive-raised sources.
One should never underestimate the strength of the human-animal bond that exists between owners and their exotic pets. An owner can be as bonded to a mouse or a snake as another owner is to a dog or horse. Just as one should never assume what an owner is willing to spend for medical care on dogs, cats, and horses, one should never assume what exotic pet owners will spend for their pets. It is not uncommon to see a devoted owner spend hundreds of dollars for a surgical procedure for a pet rat.
Some veterinary practices see primates and venomous species. Because of the dangers to humans, these veterinarians typically set “rules of engagement” regarding the care and treatment of these animals. For example, the veterinarian may only see a primate or venomous snake after hours, when all employees and clients are gone. Likewise, a veterinarian may require that an owner of a venomous snake provide in-date antivenin along with the snake.
Some veterinarians will not see large exotic cats due to safety concerns. And yes, there are people who have permits to keep them. Others will see these animals on the owner’s premises as long as handling equipment, such as squeeze cages, is provided. It is important that all employees know the clinic’s protocol for seeing primates, venomous species, and large cats.
Every state has different laws regarding which species are legal to keep as pets and which are not. It is up to the veterinarian to decide whether she will see animals that may in fact be illegal pets, and to communicate this information to the technicians and other staff.
Continuing education is an important part of a graduate technician’s professional enhancement, and its importance in exotic medicine cannot be overemphasized. What is known about the care and treatment of exotic animals is forever changing as more and more is learned. What was described as the proper diet for a particular lizard one year may be something different the next. More and more drugs are being tried in exotics. This type of cutting-edge information is often presented at conferences and in professional publications. This presents an added challenge to practices that see exotic animals because information is forever changing.
In response to this challenge, we have assembled here for the veterinary technician a survey of the most recent practices in the area of exotic animal care. Exotic animal medicine provides a veterinary technician with the opportunity to use all of his skills and knowledge in a way that has a direct benefit to the practice and to the patients. Enjoy!
The Class Aves consists of more than 8,500 species of birds and 29 orders of birds. Two orders commonly kept as pets in the United States are the Psittaciformes (parrots) (Table 2.1) and the Passeriformes (canaries and finches) (Figures 2.1, 2.2). Anatomically and physiologically there is no generic bird, meaning that each species is different in its anatomy, hematology (lymphocytes may predominate in some species), and drug metabolism. Avian medicine has many similarities to canine and feline medicine, as well as some definite differences. The similarities include use of similar, albeit smaller, equipment, similar drugs, and similar techniques. Most differences encountered in caring for birds relate to the drastically different anatomy and physiology, especially respiratory physiology, and this in turn dictates a different approach to restraint, providing air to the lungs, and supportive care. Once these differences are recognized, avian medicine is quite straightforward and rewarding.
Table 2.1. Examples of common species of birds encountered in practice.
Figure 2.1. Two scarlet macaws (Ara macao) in an outdoor aviary. This is an example of a psittacine bird, or parrot.

The anatomy and physiology of birds is drastically different from mammalian anatomy and physiology. These differences are usually due to an adaptation that helps enable flight or relates to development within an egg.
Feathers are made of keratin and are used for flight, insulation, and attracting a mate. There are various types of feathers including primaries, also known as wing remiges and tail rectrices (very large feathers that originate from the carpus and metacarpus, and pygostyle, respectively), secondaries (large feathers that originate from the radius and ulna), contour (over the body), and down feathers (produce powder down). Feathers lay in feathered tracts called pterylae, and the non-feathered tracts are called apterylae. The main shaft of the feather is called the rachis; barbs are attached to the rachis, and barbules are attached to the barbs at a 45-degree angle that hook with nearby barbules at a 90-degree angle (Figure 2.3).
Figure 2.2. A female fawn-colored zebra finch (Poephila castanotis). This is an example of a passerine, or soft billed, bird.

The very thin skin (two to four cell layers thick in feathered areas) is difficult to suture, usually requiring 4-0 or 5-0 suture. There is very little, if any, subcutaneous tissue. The feet are an exception in that they usually have thick, prominent scales in the non-feathered regions to protect them from trauma. The wing web of a bird is called a patagium. There are only two proper glands in birds, the bi-lobed uropygial (preen) gland that helps waterproof the feathers, which are absent in some birds (such as in Amazon parrots), and the ear gland, which is absent in most birds. Birds have no external ear pinna and no sweat glands. Birds bruise green because they lack biliverdin reductase, which converts biliverdin to bilirubin. Do not confuse a bright green bruise on a bird for gangrene.
Unlike mammals, birds can have a variable number of cervical vertebrae; they have eight to twenty-five instead of seven (King 1984) (Figure 2.4). Birds use their long, flexible necks to gain access to food and to reach the uropygial (preen) gland to preen their feathers. The remainder of the spine is fused in many areas to provide a stable body part for flight. A keel along the sternum provides for attachment of the large pectoral (flight) muscles. The notarium is a fusion of the first thoracic vertebrae. The synsacrum is a fusion of the caudal thoracic, lumbar, sacral, and caudal vertebrae. The pygostyle is a distal fusion of the caudal vertebrae for tail muscle attachment. The sternum has a prominent keel for pectoral muscle attachment. The pectoral girdle consists of the unique coracoid bone, that acts as a strut enabling flight, the clavicle, and the scapula. Bones of the wing from proximal to distal include humerus; radius; ulna; ulnar and radial carpal bones; and major and minor metacarpals, phalanges, and alula (remnants of a thumb). Bones of the hind limb from proximal to distal include femur, tibiotarsus, tarsometatarsus, and phalanges.
Figure 2.3. The central main shaft of the feather is called the rachis. Barbs extend from each side of the rachis at a 45-degree angle. Microscopically, barbules extend from each side of the barb a 45 degree angle. Barbules on the leading edge of a barb hook onto the barbules of the trailing edge. When birds preen their feathers, they are realigning these barbules.
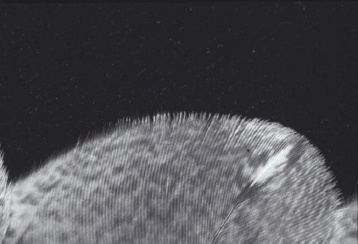
Figure 2.4. Avian skeletal anatomy.
Most important clinically is that the femur, humerus, and some vertebrae are pneumatic bones—bones filled with air—which connect directly to the respiratory tract to lighten the bones for flight. Intraosseous catheters should not be placed in pneumatic bones because any fluid administered could go directly to the lungs and drown the bird.
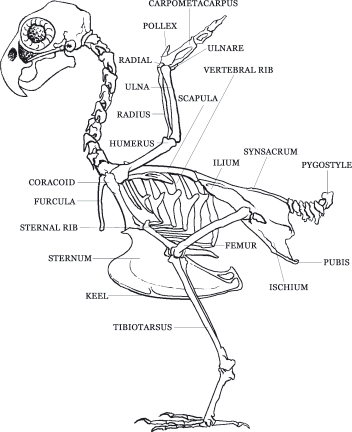
Birds, like mammals, possess a four-chambered heart, but unlike mammals, birds lack a diaphragm, therefore the apex of the heart is directly surrounded by liver (Figure 2.5). The avian heart is comparatively one and a half to two times larger than a mammalian heart. Unlike mammals, the mean electrical axis of birds is negative 90 degrees (in dogs it is positive 90 degrees). Birds do not possess lymph nodes, but they do have lymph vessels. Phlebotomy sites in birds include the right jugular vein (the right one is two-thirds larger than the left), basilic (or cutaneous ulnar) vein, and medial metatarsal vein. The cutaneous ulnar vein, as it crosses the proximal ulna, is an excellent vein for determining vein refill time; if the vein can be seen to refill this is considered slow and suggestive of dehydration or shock. Through a renal-portal system, birds can choose to shunt blood from the caudal half of the body through the kidneys first before going through the heart. Therefore, it is better to give parenteral medications in the front half of the body (i.e. give IM injections in the pectoral muscles rather than the in the leg) (Table 2.2).
Figure 2.5. Birds, like mammals, possess a four-chambered heart, but because birds lack a diaphragm, the apex of the heart is directly surrounded by liver.
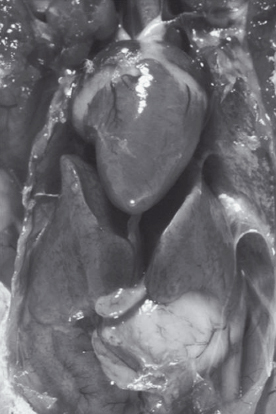
Table 2.2. Representative heart and respiratory rates for various species of birds.
Birds possess a renal portal system in which blood from the caudal half of the body may pass through the kidneys first before reaching the heart. This means that any drug administered in the caudal half of the body may go undiluted directly to the kidneys before going to the heart. Parrots have three divisions to their kidneys (cranial, middle, and caudal) and the kidneys are located dorsally in a concavity of the sacrum. Avian kidneys produce both urine (from their mammalian-type nephrons) and urates (from their reptilian-type nephrons that lack a loop of Henle). Urates consist of uric acid. Therefore, uric acid concentrations and not BUN are evaluated to determine renal function in birds.
Birds possess a large optic nerve compared to mammals. In fact, the two optic nerves together are larger than the bird’s spinal cord. Olfactory lobes are small in most birds because sense of smell is not an important sense in most birds. The eyes of a bird constitute approximately 15% of their body weight, whereas in humans they constitute 1%. The avian iris consists of voluntary, striated muscle, rather than smooth muscle as in mammals; therefore, atropine is ineffective at dilating the pupils. Birds have a well-developed third eyelid that closes over the eye in a craniodorsal to caudal ventral direction (Figure 2.6). A unique pigmented structure called the pecten, which is attached to the retina, supplies nutrients to the vitreous. Birds have no tapetum, but they have an avascular retina.
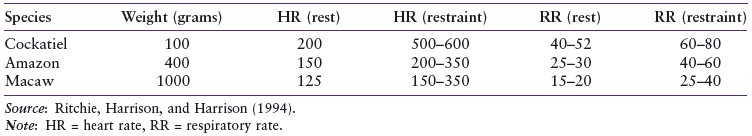
Figure 2.6. Birds have a well developed third eyelid that closes over the eye in a craniodorsal to caudal ventral direction.
The cere is an area at the base of the upper beak that surrounds the nostrils (nares) (Figure 2.7). Just inside the nares in parrots is a keratinized flap of tissue called the operculum. Birds possess an extensive infraorbital sinus; in fact, most of their head is sinus. Compared to mammals, birds have a very large trachea, allowing birds to inhale more air than do mammals. The opening to the trachea is called a glottis (Figure 2.8). Birds have complete tracheal rings; therefore uncuffed endotracheal tubes must be used to avoid pressure necrosis inside the trachea. Again, birds lack a diaphragm; therefore, they must be allowed to move their sternum up and down or they will suffocate. Old stories of birds dying right after being restrained were probably due to accidental sternal compression and secondary suffocation.
The syrinx is responsible for sound generation in the bird, not the larynx, as in mammals. Because the syrinx is just past the tracheal bifurcation, birds can still vocalize even when intubated. The path of air through the lungs goes from the trachea or air sacs to the primary bronchus to the secondary bronchus to the parabronchi to the air capillaries. Birds have air capillaries that are 3 microns in diameter, whereas mammals have alveoli that are approximately 10 microns in diameter. Therefore, birds have a comparatively greater lung surface area than mammals. Birds also have air sacs, usually nine of them, that store and warm air (Figure 2.9). Because air can go from the air sacs to the lungs, as well as from the trachea to the lungs, oxygen exchange occurs on both inspiration and expiration, increasing oxygen use in birds compared to mammals.
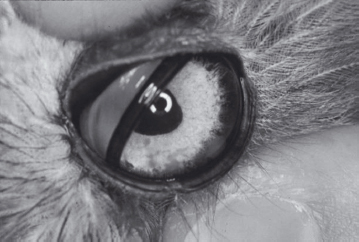
Figure 2.7. The cere is an area at the base of the upper beak that surrounds the nostrils (nares). In adult male budgerigars, such as this one, the cere is blue. In adult female budgerigars the cere is a brownish pink.

Figure 2.8. The opening to the trachea in birds is called a glottis. The glottis is usually located directly caudal to the base of the tongue in most birds. This is the glottis of a barn owl. Also note the V-shaped opening on the roof of the mouth called the choana.
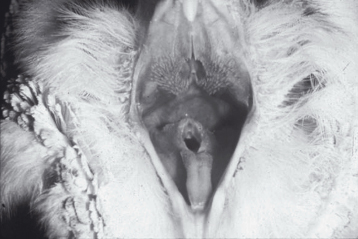
Figure 2.9. Necropsy of a parrot demonstrating clear, normal air sacs. Air sacs warm and store air. Because air from the caudal air sacs shown here go directly to the lungs, air, oxygen, or anesthesia can be delivered through a tube (air sac tube) placed into one of these air sacs.

Birds lack a diaphragm, so they possess a coelomic cavity, not an abdominal cavity. Birds do not have teeth; instead, they have a beak that is variable between species. Parrots are sometimes called hookbills because of their strong, hooked beak. The tongue is quite variable among bird species; parrots have a muscular tongue. The esophagus in birds is divided into two sections (cervical esophagus and thoracic esophagus) by an out-pouching of the esophagus called the crop (ingluvies). The ingluvies stores food and has waves of peristalsis that occur at a rate of at least one per minute. Birds possess a proventriculus (true glandular stomach) and a ventriculus (the gizzard) (Figure 2.10). Some birds possess a cecum (chickens) while others lack one (parrots). Some birds possess a gall bladder, while others lack one (parrots).
The feces of parrots contains mainly (90% or more) Gram-positive organisms (purple); waterfowl, raptors, and poultry can have mostly Gram-negative organisms (pink). Typically, passerine birds have very little bacteria in their feces, and it is Gram-positive. Clostridium spp. should not be seen in parrot feces and is characterized by a septic tank smell to the feces and the characteristic safety-pin or racket shape seen on a Gram stain (Color Plate 2.1). The cloaca is the end point for three systems: gastrointestinal, reproductive, and urinary. The cloaca is divided into three parts: the copradeum receives feces from the rectum, the urodeum receives urine and urates from the ureter and sperm or eggs from the vas deferens and uterus/vagina, respectively, and the proctodeum is the area just before the opening (vent).
Figure 2.10. Avian viscera.

The male bird possesses two intra-abdominal testis and a phallus (a rudimentary fold of tissue that is either intromittant or non-intromittant). The female bird usually possesses only one left ovary (the right ovary usually fails to develop). The female reproductive tract consists of an infundibulum, magnum, isthmus, uterus (shell gland), and a very short vagina. Parrots are not usually sexually dimorphic; therefore, surgical sexing or blood sexing must be performed to determine the gender of a bird. Surgical sexing involves visualizing the gonads and reproductive tract via a rigid endoscope placed in the abdominal air sac. Blood sexing involves evaluating 0.2 ml of blood via an ELISA test for a heterogamete (female is ZW) or homogamete (male is ZZ).
Plate 2.1. Clostridial overgrowth is apparent in this fecal Gram stain from a parrot. Clostridium shown here is a large Gram-positive rod with no spore, a clear central spore (safety pin shape), or clear end spore (racket shape).
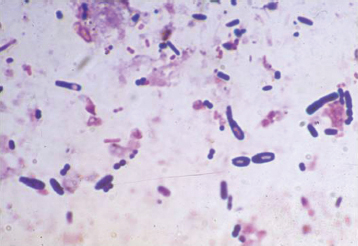
Plate 2.2. A Diff-Quick stained blood smear from a parrot. Note that birds have nucleated red blood cells. The cell at the twelve o’clock position is a lymphocyte, the two o’clock position is a monocyte, the six o’clock position is a heterophil (like a neutrophil), and the nine o’clock position is a normally occurring nucleated thrombocyte.

The blood glucose of birds is twice that of mammals. Birds possess heterophils instead of neutrophils; they are called heterophils due to the different, eosinophilic staining of the rod-shaped cytoplasmic granules. Birds, like reptiles, have nucleated RBCs and thrombocytes (not platelets). Some parrots are lymphocytic species like cows (Amazon parrots, cockatiels, budgies, eclectus, etc.) Birds can show up to 8% polychromasia since their RBC lifespan is so short (thirty-eight days compared to the 120 days of most mammals) (Color Plate 2.2).
Species of birds kept as pets come from all over the world. Their diets are as varied as they are and the environments they come from. The specific dietary requirements for all these species are not well known.
Color Plate 2.3