

Table of Contents
Preface
Chapter 1. Physical Extraction Operations
1.1. Solid-solid and solid-fluid separation operations
1.2. Separation operations of the components of a fluid phase
1.3. Bibliography
Chapter 2. Hydrometallurgical Operations
2.1. Leaching and precipitation operations
2.2. Reactor models based on particle residence time distribution functions
2.3. Reactor models based on the population balance equation model
2.4. Solvent extraction operations
2.5. Bibliography
Chapter 3. Gas-solid and Solid-solid Reactors and Particle Conversion Operations
3.1. Overall presentation of gas-solid and solid-solid reactors
3.2. Gas-solid reactor hydrodynamic behavior and heat transfer
3.3. The performance equations of gas-solid packed-bed reactors
3.4. The performance equations of fluidized-bed reactors
3.5. Solid-solid reactors
3.6. Bibliography
Chapter 4. Blast Furnaces
4.1. Overview of blast furnaces
4.2. Iron blast furnace
4.3. Ferromanganese blast furnace
4.4. Zinc blast furnace: the Imperial smelting furnace
4.5. Lead blast furnace
4.6. Bibliography
Chapter 5. Smelting Reduction Operations
5.1. Overview of smelting reduction operations
5.2. Production of (iron) “hot metal” by carbothermic smelting reduction
5.3. Tin and zinc smelting reduction operations
5.4. Magnetherm process
5.5. Bibliography
Chapter 6. Steelmaking Operations
6.1. Overview of steelmaking operations
6.2. Hot metal pretreatment operations
6.3. The hot metal converting operation
6.4. Stainless steelmaking operations
6.5. Ultra-low carbon steel-making operation
6.6. Bibliography
Chapter 7. Sulfide and Matte Smelting and Converting Operations
7.1. Overview of the operations and processes
7.2. Flash-smelting operations and processes
7.3. In-bath smelting and converting operations in bottom-blown
7.4. In-bath smelting and converting operations in top-blown converters
7.5. Top-submerged lance (TSL) blown converters: Ausmelt/Isasmelt process
7.6. Bibliography
Chapter 8. Electric Melting and Smelting Furnaces
8.1. Introduction
8.2. Performance of electric furnaces
8.3. Electric arc melting furnaces
8.4. Electric smelting reduction furnaces
8.5. Consumable-electrode remelting furnaces
8.6. Bibliography
Chapter 9. Molten Salt Electrolysis Operations
9.1. Overview of molten salt electrolysis operations
9.2. Chloride electrolysis
9.3. Reduction of alumina by electrolysis
9.4. Electro-reduction of metal oxides and deoxidation of metals by molten salt electrolysis
9.5. Bibliography
Chapter 10. Extractive Processing Routes
10.1. Features of extractive processing routes
10.2. Hot metal, steel and ferroalloys
10.3. Aluminum (gallium)
10.4. Copper and other valuable metals
10.5. Nickel (cobalt)
10.6. Zinc (cadmium, indium, germanium, gallium)
10.7. Lead (silver, gold, bismuth)
10.8. Tin
10.9. Magnesium
10.10. Titanium, zirconium and hafnium
10.11. Chromium
10.12. Molybdenum and tungsten
10.13. Niobium and tantalum
10.14. Gold
10.15. Metals belonging to the PGM
10.16. Silicon
10.17. Bibliography
List of Symbols
Index
Summary of Volume 1
Summary of Volume 2

First published 2011 in Great Britain and the United States by ISTE Ltd and John Wiley & Sons, Inc.
Apart from any fair dealing for the purposes of research or private study, or criticism or review, as permitted under the Copyright, Designs and Patents Act 1988, this publication may only be reproduced, stored or transmitted, in any form or by any means, with the prior permission in writing of the publishers, or in the case of reprographic reproduction in accordance with the terms and licenses issued by the CLA. Enquiries concerning reproduction outside these terms should be sent to the publishers at the undermentioned address:
ISTE Ltd
27–37 St George’s Road
London SW19 4EU
UK
www.iste.co.uk
John Wiley & Sons, Inc.
111 River Street
Hoboken, NJ 07030
USA
www.wiley.com
© ISTE Ltd 2011
The rights of Alain Vignes to be identified as the author of this work have been asserted by him in accordance with the Copyright, Designs and Patents Act 1988.
Library of Congress Cataloging-in-Publication Data
Vignes, Alain.
Extractive Metallurgy 3/ Alain Vignes.
p.; cm.
Includes bibliographical references and index.
ISBN 978-1-84821-292-3
1. Metallurgical plants. 2. Metallurgy. 3. Extraction (Chemistry) I. Title.
TN675.5.V54 2011
669.028--dc22
2010048941
British Library Cataloguing-in-Publication Data
A CIP record for this book is available from the British Library
ISBN 978-1-84821-292-3
Extractive metallurgy is the art of extracting metals from their ores and refining them.
This book deals with the processes, operations, technologies and processing routes of extractive metallurgy, i.e. the (production) extraction of metals from ores, concentrates (enriched ores), scraps and other sources and their refining to liquid metals before casting or to solid metals.
In many books dealing with metallurgy, the introduction starts by recalling the steps of the progress of metallurgy. These steps, according to and since Lucrèce, are identical to those of human progress: the copper age, the bronze age, the iron age, the silicon age1. According to Mohen2, the considerable role attributed to the three principal metals in the development of human societies must not be overstressed or overvalued. It is nonetheless true that “metallurgy is the most advanced prehistoric manifestation of the mastery of natural resources” (Mohen). Extracting copper from its ore dates back to the middle of the fifth millennium before our age and extracting iron from its ore dates from the beginning of the second millennium before our age.
The winning (production) of metals and alloys today is still one of the basic industries of the transformation of matter. Metals and alloys still are essential resources for metallic, mechanic, electromagnetic, electric and even electronic industries (silicon is treated as a metal).
This industry is characterized by:
– Production (of primary metal) ranging from 1,345 million tons (Mt) of steel a year to 138,000 tons of titanium, in 20073.
Table 1. World metal production in 2007

– Very high growth rates in the years 1950 to 1973, and again since 2000. The production of steel was 200 million tons in 1950. The production of aluminum increased from 2 million tons in 1950 to 10 million tons in 1973, reaching 38 million tons in 2007. If in developed countries the growth in terms of tonnage has strongly slowed in recent decades, this is due to a smaller consumption of these products owing to the increase in mechanical and physical properties of the materials and parts forged from these materials, thus requiring less material for the same usage. However the annual production of steel in China increased from 182 million tons in 2002 to 489 million tons in 20074.
– Production costs varying by a factor of 20 to 25 between steel and titanium. The three principal costs in metal production are investment, ore and energy consumption. The energy consumption is about 20 GJ/ton of steel, 80 GJ/ton of aluminum and 160 GJ/ton of titanium. Hence the permanent research into improvements of the processes or operations and/or the development of new processes.
– Very high recycling rates. Recycled steel represents 46% of iron sources in worldwide steel production. The “electric furnace processing route” produces 35% of steel. It uses 75% less energy than the integrated route. The recycling rate of aluminum represents 25% of total production and the energy consumption from recycled aluminum represents 5% (energy reflow) of energy consumption from the ore. The production of primary zinc is 7.4 million tons and from recycled zinc is 2.1 million tons. In the case of lead, the production from recycled lead is greater than 50%.
– Very high quality products with degrees of purity (i.e. contents of harmful impurities) for the finished products, comparable to the purity of materials for electronics and with very narrow concentration ranges of the alloying elements, to obtain physical or mechanical properties with very small dispersions. For metal castings reaching 300 tons, steel grades with carbon content of less than 25 ppm, and sulfur and phosphorus content of less than 20 ppm or even 10 ppm can be guaranteed. The impurities in liquid aluminum after electrolysis and refining are <3 ppm for Li, <1 ppm for Ni and <1/10 ppm for H. The contents of each impurity in copper for electric wire must be <1 ppm. Achieving these chemical performances coupled to research into the lowest energy consumption requires perfect mastery of the process and thus a profound knowledge of its technology.
– The energy consumption and reduction of pollution (rejected CO2, SO2 and dust) from the production of metals have become major objectives, leading to the development of new processes or product lines.
– Non-ferrous metal ores often have very low contents of many rare or noble metals, whose extraction and recuperation often constitutes essential steps for the global production economy. Such extraction requires very complex processing routes for recovering rare or precious metals.
Often the metal can or could be produced via several processing routes. The industrial processing routes for a given metal are to a large extent dependent on economic considerations, i.e. the cost of raw materials, cost of energy, cost of equipment and market conditions.
The raw materials for the production of metals and alloys are the ores on one hand and recovered and recycled products on the other:
– the ores. The ores of Sn, Fe, Mn, Cr, Al, Ni are oxides. The ores of many non-ferrous metals, e.g. Cu, Ni, Pb, Zn, Cd, Mo, are sulfides;
– the recycled metals (Fe, Al, Cu, Zn, Pb);
– the steel plant dust containing metals or oxides (Zn, Cd, Pb);
– the residues from leaching operations, e.g. the red muds, a residue containing titanium, vanadium, gallium produced by bauxite leaching during the Bayer process, the gold cyanide sludge;
– the drosses, slags and scoria treated to recover rare metals or to eliminate harmful components.
The operations of mineralogy are known as ore-dressing. In the general case, the ore must be concentrated to free it from minerals of no value, called the gangue, whose main components are oxides (SiO2, Al2O3, CaO, MgO). This is done using physical operations: grinding (comminution) or fragmentation of the ore to small sizes to allow easy separation, then separation by sedimentation and enrichment by flotation, magnetic sorting etc., leading to a raw material enriched in components.
The operations of extractive metallurgy treat ores, concentrates and recycled metals. These are mixtures of oxides or sulfides. The processing routes of the ore’s treatment, raw or enriched, together with the technologies used in these routes depend first of all on the ore’s nature and its metal content.
Thus iron ore is practically pure iron oxide (hematite or magnetite), with a content of iron of the order of 65% and several percent of silica (SiO2). The basic treatment will be the direct reduction of the iron oxide.
Alternatively, the ores can be treated to give an essentially pure chemical compound of the metal and this compound may be converted to give the metal. For example, aluminum’s ore (bauxite), is composed of alumina (Al2O3, 30–60%), iron oxide (Fe2O3, 1–30%) and silica (1–10%). The first phase of the ore’s treatment will be the separation of these oxides to obtain the pure alumina, which will be reduced in a second phase by electrolysis in molten salts.
The copper sulfide ores, whose copper content is very low, exceptionally reaching 5%, undergo processes of mineralogy (flotation) to obtain concentrates containing Cu (20–25%), Fe (30%) and S (30%). The separation of copper sulfide from iron sulfide via a selective roasting constitutes the first step of the treatment. The second step is a copper converting.
Zirconium ore, zircon (silicate of zirconium and hafnium, i.e. ZrSiO4 and HfSiO4), is converted into gas chlorides whose separation is possible before the reduction of zirconium chloride to very pure zirconium.
Extraction and refining operations may be carried out by pyrometallurgical, hydrometallurgical, halide and electrometallurgical processes:
– pyrometallurgy involves processes carried out at high temperatures divided into:
– primary pyrometallurgy, which converts the ore or concentrate to impure metal generally in liquid form. The main operations are oxide reduction, sulfide roasting, smelting and converting;
– secondary pyrometallurgy is the treatment of the liquid metal, obtained either directly in the first step or by remelting metallic recycled products. It consists of several refining operations, mainly the removal of harmful elements left in the liquid metal (deoxidation, dehydrogenation, etc.) and addition of the alloying elements;
– hydrometallurgy consists of operations of primary metallurgy performed in aqueous solutions, at relatively low temperatures and often under high pressure, such as leaching, precipitation and solvent extraction;
– hydroelectrometallurgy consists of salt electrolysis in an aqueous solution, yielding the metal in a solid state. Electrorefining constitutes a refining process of the metal obtained in a first electrolysis;
– pyroelectrometallurgy consists of processes employing electrolysis (reduction), either of mattes or oxides (e.g. Al2O3) or chlorides (e.g. MgCl2) into molten salts, yielding the metal in a liquid state;
– chlorometallurgy consists of the following processes:
The upholding into operation of an existing processing unit, the improvement of an industrial operation, the implementation of a new technology (not formerly used in the unit) and the development of a new process all fall within technical considerations, as well as economic considerations. In this series, economical considerations will not be discussed, for obvious reasons, but sound economic decisions rest on in-depth technical analyses of the processes and operations. Such in-depth analyses are based on process engineering principles. These methods use mathematical models allowing us to simultaneously take into account the elementary processes and their couplings5. These mathematical models are sets of fundamentally-based differential equations derived from thermodynamics, kinetics, heat flow, fluid flow, mass transfer and electromagnetic phenomena. Modeling will thus be at the heart of all the analyses here. The solutions to these differential equations, via analytical or numerical methods, allow us to achieve sound quantitative previsions. Analytical solutions of these equations of partial derivatives have been established in numerous instances, but only for specific cases. They are nonetheless interesting as they reveal the influence of certain factors or parameters on the processes. This leads to very useful dimensionless numbers. These analytical solutions and the dimensionless equations are presented and used in these volumes. For the numerical methods of the solution of equation systems, the reader is referred to specialized publications.
The subject of extractive metallurgy is also addressed in two other publications written by myself and published by ISTE. The first volume, Basic Thermodynamics and Kinetics deals with the fundamentals of thermodynamics and kinetics of the extraction processes. The second volume, Metallurgical Reaction Processes, deals with the extraction and refining unit processes. This final volume, Processing Operations and Routes, deals with the operations and technologies used in industrial production and industrial processing routes, i.e. the combination of steps or operations used to convert the available ore to metal, illustrated by flowsheets.
This book is intended not only for students of metallurgical and mechanical engineering who want to acquire the bases of this technology, decreasingly taught in universities and engineering schools, but also for engineers confronted with a new production problem, either directly (management of a industrial operation or development of a new process) or indirectly (in the definition of a materials’ specification).
It is conceived to be accessible to any student or engineer with general chemistry and physics training. It only necessitates elementary knowledge in chemistry, thermodynamics and chemical kinetics. One of the objectives of this book is to allow the easy consultation of books and technical publications dealing with this field.
This book is the result of my chemical engineering training, courses taught in the Écoles des Mines of Nancy and Paris (France), visits to industrial plants, research performed in collaboration with industry, studies and common work as a consultant and as an industrialist in direct contact with numerous producers of metallic parts. I would like to thank, more particularly, engineers from the research centers of Arcelor-Mittal (IRSID), Alcan (ex-Péchiney), Cezus and Eramet for their advice and authorized opinions.
I would most particularly like to thank Professors Jean Philibert and André Pineau. Finally this book is dedicated to Professors Pierre Le Goff and Pierre-Marie Fourt.
Alain VIGNES
February 2011
1 S.L. SASS, The Substance of Civilization: Materials and Human History from the Stone Age to the Age of Silicon, Arcade Publishing, 1999.
2 J.P. MOHEN, Métallurgie préhistorique, Masson, Paris, 1990.
3 US Geological Survey, Minerals Commodity Summaries and Minerals Yearbook, 2007.
4 Source: IISI (International Iron and Steel Institute).
5 J. SZEKELY, “The mathematical modeling revolution in extractive metallurgy”, Metallurgical Transactions B, Vol. 19B, p. 525–540, 1988, and H.Y. SOHN, “The coming-of-age of process engineering in extractive metallurgy”, Metallurgical Transactions B, Vol. 22B, p. 737–754, 1991.
This chapter presents the unit operations of separation of the components of a mixture. These physical operations do not involve a chemical reaction. Only the bases of these processes are presented.
Flotation (“froth flotation”) is one of the primary mineral processing operations that is a beneficiation process. Flotation is a sorting (separation) process of chemically different solid particles by using surfactants and wetting agents [FUE 07]. The principle of froth flotation is as follows: after grinding into a fine powder, the solid particles to be separated are mixed with water. A water slurry (called a pulp) is then made. This pulp is treated with reactants (surfactants) that, by being selectively absorbed at the surface of certain particles, make them hydrophobic (having a greater affinity for air than for water).
This pulp of hydrophobic and hydrophilic particles is placed in a flotation cell with impellers (see Figure 1.1.1) and air bubbles are blown through it. Air bubbles attach themselves to the hydrophobic particles and carry them to the surface if the result of the surface tension, mass and buoyancy forces is negative. They are floated out in a stable froth (concentrates) that is skimmed off and discharged of its burden.
Figure 1.1.1. Diagram of a froth flotation cell: showing pulp from the previous flotation cell; and pulp to the next flotation cell
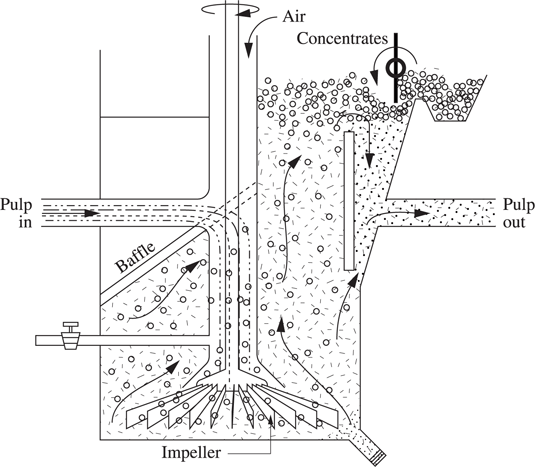
Collectors are ions of organic molecules that belong to the family of alkyldithiocarbonates or xanthates and dithiophosphates [RAO 04].
Flotation is one of the processes used for concentrating (beneficiating) complex ores, such as sulfide and carbonate ores of nonferrous metals (lead, copper, zinc, nickel). Sulfide ores can always be concentrated by flotation with good yields. Flotation is the most commonly used technique for separating minerals from their gangue (silicates are wetted by water, sulfides are non-wetted). The ore has to be subjected to a grinding until particles with a size lower than 150 μm are obtained. This operation has a major economical importance (units have a treatment capacity of more than 100,000 tons of ore per day).
Flotation also allows the separation of sulfides: in the treatment of zinc-lead sulfide ores, nickel-copper sulfide concentrates and nickel-copper mattes (see Chapter 10, Figure 10.5.2) leading to a concentrate rich in one sulfide and a concentrate rich in the other.
In the refining operations of steel and aluminum, flotation is used to remove from the liquid phase oxide inclusions formed during the deoxidation of steel (see [VIG 11b], Chapter 7, section 7.2.3) or present in [MIR 09]. This operation is performed by injection of argon bubbles that attach themselves to the inclusions.
The removal of sulfur S°, which forms in the leaching of zinc sulfides, is performed by flotation (see [VIG 11b], Chapter 1, section 1.5.2.2).
Settling under gravity is the natural separation of solids that are suspended in a liquid. Settling can be accelerated by coagulation of the particles with flocculating agents.
The gas-solid separation is carried out in cyclones (see Figure 1.1.2). The solid particles carried along by a gas flowing at a high rate, due to the centrifugal force, end up on the walls of the cyclone during a downward helical journey and fall to the lower conical part, whereas the gas escapes from the higher part.
For liquid-solid suspensions, the separation by centrifugation is obtained by rotating the suspension.
Figure 1.1.2. Gas-solid separation in a cyclone

Ordinary filtration is used in hydrometallurgical operations. For aqueous suspensions, the separation of the particles resulting from a precipitation is obtained by filtration, which is carried out by passing the slurry through a natural or synthetic cloth that will not permit passage of solids. The cloth should offer minimal resistance to water flow and act mainly as a support for the deposited solids that form the effective filter bed. The water may be forced through the filter under pressure or drawn through by suction.
The presence of non-metallic inclusions in cast iron, steels and aluminum alloys is very detrimental to product quality. These very fine oxide inclusions (of 1–3 μm) are formed when the steel is deoxidized with various deoxididizers, such as aluminum, silicon, etc. They do not separate into the slag phase during these refining operations in spite of their low density. Filtration of liquid metals prior to casting is used to remove inclusions (see [VIG 11b], Chapter 7, section 7.2.3).
The ceramic (alumina) filters are made either of a bed of spherical particles or of a monolith bearing parallel cylindrical pores with a diameter of about 1 mm. The pore diameter in a deep bed filter can be 100 times as large as the filtered particle diameter. The particles are captured by adsorption on the pore walls throughout the entire depth of the filter. The flow rate of the liquid metal in these pores is thus a significant parameter for the efficiency of these filters. The ceramic (alumina) used allows the filtration of aluminum alloys (see Figure 1.1.3), copper alloys, cast iron (see Figure 1.1.4) and of super-alloys.
Figure 1.1.3. Pechiney deep-bed filter [CLE 95]

Figure 1.1.4. Ceramic filter for cast iron (Daussan Group document)

A filter, such as the one presented in Figure 1.1.3, with a height of 1.5 m and a global surface of 2.7 m2 can treat 25 tons of aluminum per hour. It contains 2.5 tons of liquid metal [BRE 95].
Condensation allows separation of components of a gaseous phase by cooling. Condensation is carried out in zirconium and titanium metallurgy in order to separate the gaseous chlorides obtained by carbochlorination of the ores (see Chapter 10, Figure 10.10.1).
In zirconium metallurgy, after the Kroll reduction that produces a pseudo Zr-Mg alloy, vacuum distillation producing a vaporization of magnesium allows the separation of magnesium and magnesium chloride from titanium or zirconium, leaving a sponge (see Chapter 10 Figure 10.10.1).
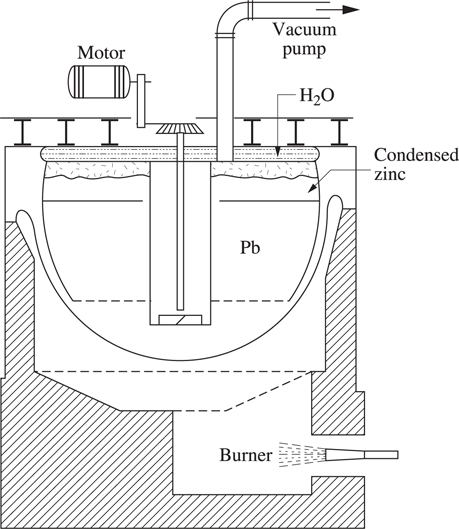
Lead bullion is refined (see Chapter 10 Figure 10.7.1) using a vacuum distillation operation, to remove zinc whose content reaches ≈0.55%. At 600°C, under a vacuum of 0.05 mbar, the volatilized metallic zinc condenses on the higher part of a tank cooled down by water (see Figure 1.2.1). A mechanical vacuum pump produces a residual pressure of about 0.05 mbar. A temperature of 600°C, a stirring time of five hours reduces the zinc content in lead from 0.50% to 0.05%.
Figure 1.2.1. Lead bullion refining in a batch vacuum dezincing plant. The distance between the evaporating and condensing surfaces is about 30 cm [DAV 00]
After cooling, a two-component liquid phase is separated into two liquid phases. This is liquation. One of the phases is rich in component A and the other is rich in component B when the phase diagram of the A-B binary solution presents a miscibility gap.
Liquation is also an operation in which impurities in a metal are removed by cooling the mixture to just above the melting point of the pure metal. Where the solubility of the impurity decreases and precipitation of this impurity or a compound occurs.
This operation is used in zinc metallurgy, in the pyrometallurgical processing route based on the Imperial smelting process, after zinc condensation by lead to separate lead from zinc (see Chapter 4, Figure 4.4.1 and Chapter 10 Figure 10.6.1). The zinc-lead equilibrium diagram (see [VIG 11a], Chapter 3) presents a miscibility gap. By lowering the temperature of the liquid phase down to 430–440°C, two liquid phases form; one of the phases is rich in zinc and contains 0.9% lead and the other is rich in lead and contains 2% zinc. The phase rich in zinc contains some iron and the solubility of iron in zinc significantly decreases when the temperature is reduced from 800°C to 419°C. By cooling, we obtain a liquid very rich in zinc and a solid component: phase ζ (whose formula is FeZn13). This operation is carried out by keeping zinc in a reverberatory furnace with a sloping hearth for 24 to 48 hours at a temperature of 430–440°C. Lead settles down, as do the FeZn13 crystals, called “zinc matte”. Three products are thus obtained:
– refined zinc with 0.9% lead;
– lead with 5–6% zinc;
– the “matte”.
This operation is carried out for many other metals. In the extraction of tin by smelting-reduction, we obtain tin with 2% iron (see [VIG 11b], Chapter 7, section 7.6 and Chapter 10, Figure 10.3.1 of this volume). Refined tin is obtained by liquation. By cooling down the tin bath, precipitation of FeSn2 compounds occurs. The solid precipitates, having a density close to that of tin, form a dross whose separation from liquid tin is performed by filtration or centrifugation. During this operation, other impurities (Cu, As, Sb) that also form inter-metallic components are also partly removed.
In lead refining, copper is the first impurity to be removed from lead bullion. The “decoppering” is performed by liquation (see [VIG 11b], Chapter 7, section 7.5.1 and Chapter 10, Figure 10.7.1 of this volume). The lead bullion is melted and held just above its melting point. The solid copper precipitates and rises to the surface, forming a dross that is skimmed off.
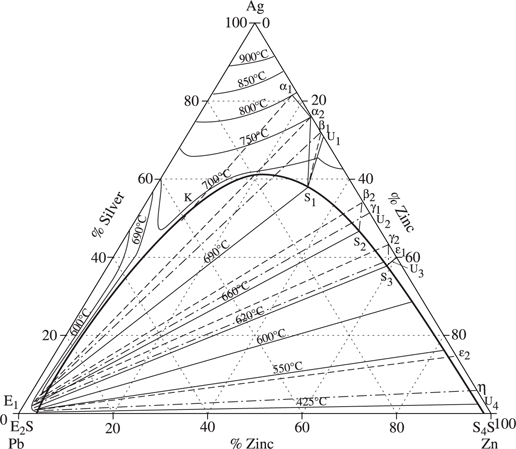
The recovery of silver and gold from lead bullion, known as “lead desilverizing” involves liquation by the Parkes process (see [VIG 11b], Chapter 7, section 7.5.4 and Chapter 10, section 10.7, Figure 10.7.1 of this volume). This is performed by addition of zinc to the (Pb + Ag + Au) solution at 480°C. The phase diagram of the Pb-Zn-Ag system (see Figure 1.2.2) shows that the system is split into two phases, with silver being concentrated in liquid zinc. The temperature of the melt is gradually lowered to below 419.5°C, at which point the zinc (now containing all the silver and gold) begins to solidify as a crust on the surface of the lead and can be skimmed off.
Figure 1.2.2. Lead-zinc-silver ternary phase diagram [WIL 80]
Distillation is a separation operation of the components of a liquid phase whose boiling points are not too close to each other. The simplest equilibrium diagram for two-component system is composed of a liquid-phase region, a gas-phase region and a two-phase liquid + gas region. The temperature-composition curves for each phase in two-phase equilibrium are the two curves that separate the single phase from the two-phase region (see Figure 1.2.3). At temperature T2, for instance, the liquid phase is richer in component B and the gas phase is richer in component A. The principle of distillation relies upon this fact.
Separation is carried out in a distillation column with trays above each other, along which a temperature gradient is imposed (see Figure 1.2.4). A layer of liquid is held on each tray and bubbles of the gas phase pass through. At each tray, the liquid phase and vapor phase in equilibrium have different compositions. There is then extraction from the liquid phase of one or two of the most volatile components, which are carried out by the gas bubbles and absorbed by the liquid phase of one or two components of the gaseous phase with the highest boiling points [PET 04].
Figure 1.2.3. Liquid-vapor phase diagram of a binary system

Figure 1.2.4. Tray distillation column

In this operation, the liquid mixture to be distilled is introduced to the column at an intermediate level. The vapor phase circulates upwards in the column as bubbles through the liquid layer of each tray; it carries away the most volatile component(s). The liquid phase circulates downwards due to gravity and becomes richer in the components whose boiling points are the highest. A fraction of the liquid at the bottom of the column is removed, as residue; another part is vaporized in the boiler. This fraction forms the vapor phase required for distillation. The vapor at the head of the column is condensed; some of the condensates are carried back to the head of the column and form the liquid phase that passes goes down the column.
This operation is carried out in the INCO pressure carbonyl process of nickel production, where nickel is converted into nickel carbonyl, Ni(CO)4 (see [VIG 11b], Chapter 7, section 7.9 and Figure 7.9.1). Distillation is used to separate nickel carbonyl Ni(CO)4 (TE: 43°C) from iron carbonyl Fe(CO)5 (TE: 102.8°C). Ni(CO)4 is released at the head of the column.
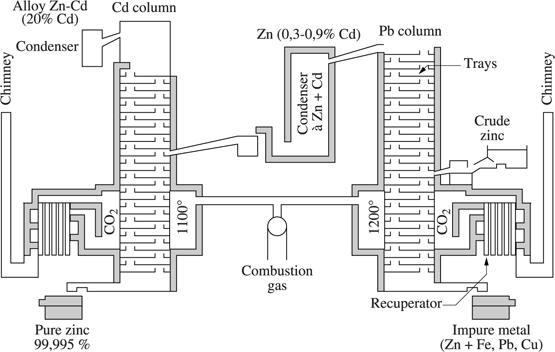
Zinc, obtained by pyrometallurgy, contains always lead, cadmium, (see [VIG 11b], Chapter 7, section 7.7). Zinc refining is performed by “fractional distillation”, in a unit consisting of four parallel working columns (see Figure 1.2.5). Each column has between 56 and 58 silicon carbide (SiC) trays with holes that allow vertical counter-current flows of rising vapor and descending molten metal zinc between the trays [HAN 00]. Crude zinc is inserted at 580°C into the middle of the lead column where the less volatile elements, such as iron, copper and lead, are removed along with half the zinc at the bottom of the column. This metal feeds a liquation furnace where the Zn-Pb separation is carried out. The zinc thus recovered is reheated to 600°C and feeds a second re-boiling column (not represented in the figure) where zinc vapor with a purity of 99.995% collects at the top of the column. The vapor passing out of the top of the lead column containing about 0.3–0.9% cadmium, is condensed and fed into the cadmium column.
After condensation, the vapor passing out of the top of the cadmium column, enriched in cadmium (20% Cd−80% Zn) is fed into a second cadmium column (not represented in the figure), at the top of which cadmium, with a purity of 99.997% or more, is obtained. The metal at the bottom of the cadmium column is zinc with a purity of 99.995%.
Figure 1.2.5. Refining of zinc: distillation unit for the separation of zinc, cadmium and lead (New Jersey zinc distillation process)[HAN 00]
When the components of a liquid phase have boiling temperatures very close to each other, extractive distillation can be used to separate them by adding a solvent in which one of the components predominantly dissolves. The distillation unit consists of two columns. The distillation column (see Figure 1.2.6) is fed with the mixture. The solvent is introduced in the column above the feed level. It has a high boiling point, remains in the liquid state and absorbs, as it moves downwards, the less volatile component. The more volatile component is obtained at the top of the column. The mixture of the solvent and less volatile component passes from the bottom of the extractive distillation column and is distilled in the second regeneration column. The purified solvent obtained at the bottom of this column is recycled in the first column.
Figure 1.2.6. Schematic representation of an extractive distillation unit

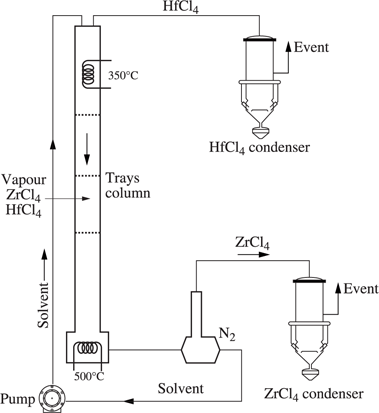
Extractive distillation is carried out in zirconium metallurgy to separate zirconium and hafnium chlorides (see Chapter 10, Figure 10.10.1). The separation of these chlorides is difficult as both components have similar properties; however the hafnium content in the ore has to be reduced from a few percent to 100 ppm in the metal, which represents an extraction factor of about 1,000. The Cezus process is a continuous process of separation of ZrCl4 and HfCl4 by extractive distillation using a mixture of molten salts, KCl-AlCl3, at a temperature of 350°C under atmospheric pressure (see Figure 1.2.7).
The zirconium and hafnium chloride vapor is preheated to 500°C and is injected at mid-level into the tray column. The ascending vapor flows counter-currently with a descending solution of potassium and aluminum chlorides, KAlCl4 (AlCl3-KCl), which are used as the solvent. This solution becomes richer in ZrCl4 and the ascending vapor becomes richer in hafnium chloride, which is released at the head of the column. At the bottom of the column, the solvent enriched in zirconium chloride passes through a boiler at 500°C where the zirconium chloride is vaporized and carried along by a neutral gas before being recovered by condensation. The solvent recovered is injected back into the head of the column.
Figure 1.2.7. Cezus process unit of extractive distillation of ZrCl4/HfCl4 chlorides (French patent 73 40395, 2 250 707, November 14, 1973)
[ALI 85] S. ALI, R. MUTHARASAN, D. APELIAN, Metallurgical Transactions B, Vol. 16B, p. 725, December 1985.
[BRE 95] R. BREMOND et al., La Revue de Métallurgie, CIT/Science et génie des matériaux, pp.593–599, May 1995.
[CLE 95] G. CLÉMENT, “The Pechiney deep bed filter: technology and performance”, in J. EVANS (ed), Light Metals 1995, The Minerals, Metals and Materials Society, pp. 1253–1263, 1995.
[DAV 00] T.R.A. DAVEY, Lead-Zinc 2000, J.E. DUTRIZAC et al. (eds.), TMS, pp. 617–636, 2000.
[FUE 07] M.C. FUERSTENAU et al., Froth Flotation, a Century of Innovation, Society for Mining, Metallurgy and Exploration, Littleton, United States, 2007 (www.smenet.org).
[HAN 00] G. HANKO et al.: in J.E. DUTRIZAC et al. (eds.), Lead-Zinc 2000, TMS, pp. 481–496, 2000.
[MIR 09] O. MIRGAUX, D. ABLITZER, E. WAZ, J.P. BELLOT, Metallurgical and Materials Transactions B, Vol. 40B, pp.363–369 363, June 2009.
[PET 04] F.B. PETLYUK, Distillation Theory and its Application to Design of Separation Units, Cambridge Series in Chemical Engineering, Cambridge, 2004.
[RAO 04] S.R. RAO, Surface Chemistry of Froth Flotation, Vol. I and II, Academic Press, New York, 2004.
[VIG 11a] A. VIGNES, Extractive Metallurgy 1: Basic Thermodynamics and Kinetics, ISTE Ltd, London and John Wiley & Sons, New York, 2011.
[VIG 11b] A. VIGNES, Extractive Metallurgy 2: Metallurgical Reaction Processes, ISTE Ltd, London and John Wiley & Sons, New York, 2011.
[WIL 80] G.M. WILLIS, Lead-Zinc-Tin 80, TMS-AIME, pp. 457–460, 1980.
The reactors, their design, size (scale-up) and process control (operation) for the three basic hydro-metallurgical operations — leaching, precipitation and solvent extraction — are dealt with in this chapter.
Leaching and precipitation operations are carried out in two types of reactors: bubble columns (also called air lift reactors, free-air-lifts or air-agitated Pachuka tanks); and the continuous stirred tank reactor (CSTR) and autoclave
In the bubble columns and Pachuka tanks used for leaching operations, a discontinuous gas phase in the form of bubbles is injected into a continuous phase, the slurry, in order to stir the liquid phase and keep the solid particles in suspension. Bubble columns can be single-staged, multi-stage, batch or continuous. Particle suspension is the most important parameter in the design of energy-efficient Pachuca tanks.
Several investigations on particle suspension have been carried out in bubble columns in the presence and absence of a draft tube [ABR 92]. Bubble columns operate with height/diameter ratio as high as 10, superficial air velocities (0.1–1 M/s) and dilute slurries containing only 5–10 wt pct particles. An expression of the critical air velocity for off-bottom suspension, VSGm, has been established by Narayanan [NAR 69].
In order to obtain a more homogeneous suspension, systematic circulation of the liquid induced by the gas current injected in a siphon (full center column, see Figure 2.1.1) is required. Pachuka tanks range in diameter from 5–10 m, with a draft tubeto- tank diameter ratio of the order of 0.1. They operate at low air velocities (10−2 m/s), the slurry containing 50–60 wt pct solids. The hydro-dynamic operating conditions of these reactors have been studied in detail [MEH 01, ROY 98, SHE 89]. An exhaustive review dealing with the design and dimensioning (sizing) of these reactors has been written by Shah [SHA 82].
Figure 2.1.1. Pachuca tanks: air-agitated slurry reactor with a full-length draft tube (fullcenter-column) showing the fluid flows [ROY 98]

CSTRs operate with a continuous feed, which is either a slurry to be leached or a solution in which solid particles will be produced by precipitation. The solid particles are maintained in suspension by mechanical stirring.
For oxidizing pressure leaching, the horizontal autoclave, operated in a continuous mode, is generally the equipment of choice [BER 91]. Autoclaves are usually made of several compartments (see Figure 2.1.2). Each compartment is equipped with a mechanical stirrer to keep the solid particles in suspension. Injection of air, oxygen or other gases occurs in every compartment. The gas phase is continually vented to remove inert gases, thus maintaining the desired partial pressure of oxygen. We can also notice the presence of coils for heat exchange. Each compartment is a “CSTR” with feed and discharge of the suspension in steady state. The slurry cascades from one compartment to the next. The composition of the leaching solution, which is considered as being homogeneous in every compartment, varies from the first compartment to the last one. Especially the concentration of one of the leaching reagents (usually an acid or H+ ion or a base or OH- ion) decreases from one compartment to the next, and thus different reactions between the first compartment (leaching) and the last compartment (for instance, precipitation) may occur (see [VIG 11b], Chapter 1, sections 1.2.2.4 and 1.3.1.3). When there is injection of an oxidizing gas, the concentration of the oxidizing agent in the aqueous phase can be considered as being constant in every compartment; thus the oxygen potential or oxidizing capacity is constant. The acidity or the basicity, however, decreases between one compartment and the next.
Figure 2.1.2. A four-compartment horizontal autoclave with mechanical stirring, gas injectors and heat exchangers

Acidic and oxidizing leachings of oxides and sulfides (see [VIG 11b], Chapter 1, sections 1.2.2. and 1.2.3) are carried out either at atmospheric pressure (T ≈ <100°C) or at higher temperature up to 250–270°C; the pressure in the reactor has to be higher than the vapor pressure of water (40 atm or 4,000 kPa at 250°C). At this temperature, the density of water is equal to 0.8 g/cm3 and therefore an autoclave filled with 80% of a liquid phase at a regular temperature will be entirely filled (100%) by the aqueous phase in the liquid state. The volume of liquid in the autoclave is usually maintained at 65–70% [BER 91, CAM 99].
The aim for these leaching operations is to obtain a dissolution of ore or concentrate particles that is as complete as possible. For precipitation operations, the aim is to obtain a distribution of the particle sizes that is as narrow as possible around a fixed mean value. The sizing (or scale-up) of these reactors for a fixed feed rate of the suspension, or conversely the setting of the operating conditions (feed rate of the suspension, flow rate of the oxidizing gas, temperature and pressure) to obtain the highest possible fractional conversion in a given reactor requires modeling.
NOTE 1.– These models, even the most sophisticated ones, relying on numerical methods provide in the best cases the orders of magnitude and trends. They are useful, however, as they can give some information on the influence of the variation of operating parameters on the evolution of a system and this is why they are presented in this section.
In the case of a homogeneous reaction, this reactor is characterized by a uniform composition of the reactive mixture throughout the available volume. If the reactor is sufficiently well-mixed, the discharge has the same composition as the steadystate composition of the solution in the reactor. The ideal residence time distribution of an elementary volume of fluid in a steady state that is well mixed in a flow reactor is given by [LEV 72, PET 91]:
[2.1.1] 
where  is the mean residence time of an elementary volume:
is the mean residence time of an elementary volume:
[2.1.2] 
where Vr is the reaction mixture volume; Q(s) is the discharge volumetric flow rate; G(discharge ) is the discharge mass flow rate of the reacting mixture; and ρ is its density.
This residence time distribution shows that all residence times are a priori possible, from the instantaneous short-circuit of the input up to infinite swirling within the tank. The elementary volumes of the discharge present fractional conversion ranging from 0 to 1. As a consequence, this reactor does not yield high fractional conversions, which explains the requirement for several CSTRs placed in cascade (or series). Industrial units are therefore made of several reactors placed in series.
For a series j of equal-sized ideally-stirred tanks, the distribution of residence times of the suspension volume, thus the residence times of the particles going through the autoclave, is as narrow as the number of stages is high [LEV 79, PET 91]:
[2.1.3] 
and the mean residence time for the tank-in-series  is:
is:
[2.1.4] 
where  is the mean residence time in one tank.
is the mean residence time in one tank.
The material balance for reactant A for one compartment is given by:
[2.1.5] 
where FA is the molar flow rate of reactant A and r is the reaction rate corresponding to the composition of the leach solution in the reactor, thus to the composition of the leach solution leaving the reactor, CA = CA(out). This depends on the fractional conversion (see [VIG 11a], Chapter 1, equation [1.2.14a]):
[2.1.6] 
For a first-order reaction rate, the performance equation is:
[2.1.7] 
and for a second order reaction rate:
[2.1.8] 
For a series j of equal-sized ideally-stirred tanks, and for the same reaction in each reactor, the performance equation is [LEV 72, LEV 79]:
[2.1.9] 
The modeling of a leaching operation in a CSTR can be carried out using two different approaches: a model based on the residence time distribution of the particles in the reactor (RTD model); and a population balance model, taking into account, the particle size distribution (PSD) in the feed in both models. The basics of these models and the corresponding analytical solutions are exhaustively presented in the books by Levenspiel [LEV 72, LEV 79]. Only the main results are presented in this chapter. The numerical methods for the first modeling are presented in Dixon’s article [DIX 95] and for the second modeling, in Villermaux’s book [VIL 93] and in Marchal’s article [MAR 88].
Most of these models assume a perfect mixing of the leaching solution on entering the reactor, which is the basis of the CSTR model, i.e. “the maximummixedness model”. Each fluid element entering the reactor is immediately mixed in the fluid present so that the concentration of the leaching reagent is uniform and constant in the reactor and equal to the concentration in the discharge. Each particle evolving independently of the others is in contact with a uniform solution during its residence time in the reactor.
A segregated-flow model has been developed by Crundwell [CRU 95]. It is based on the concept of segregated flows in which the material in the feed on entering the reactor is dispersed into volume elements composed of both particles and fluid. These elements remain intact and respond as batch reactors during their residence time in the reactor. The reaction of the particles with the solution results in lowering of the reactant concentration within each volume element. This lowering of the reactant concentration is a function of the residence time of the volume element (see equation [2.1.1]). This modeling is not presented in this book.
Mathematical model equations have been developed for multistage CSTR in which multisize feeds react according to the surface reaction controlling shrinking core model (see [VIG 11a], Chapter 7, section 7.2.1.1).
If we are only looking for the expression of the kinetic law of the fractional conversion of a set of particles as a function of the operating parameters, we can use the approach of the residence time distribution of particles in the reactor [DIX 95, PET 91]. If we are looking not only for the fractional conversion of a set of particles but also for their size distribution at discharge, the population balance approach is required [MAR 88].
For leaching operations, modeling based on the distribution function of the residence times is usually used. For precipitation operations, population balance model is mainly used.
In the modelings, we usually assume that distribution of the residence times of the particles is that of the volume elements of the suspension (see previous section) and is given by expressions [2.1.1] and [2.1.3]:
[2.1.10] 
where V(slurry) is the suspension volume in the reactor and G(slurry in the discharge) is the mass flowrate of the suspension.
Actually, even in reactors that are stirred enough for the fluid phase to be considered uniform, the particles settle down and are resuspended by stirrers. The distribution of the residence times can therefore be very different from the one given by expressions [2.1.1] and [2.1.3] and has to be determined experimentally
For a CSTR, the number of particles in the feed and the reactor is high enough to describe the population in statistical terms by (differential) particle-size distribution (PSD) function or particle-size density function or number density function Ψ (Rp):
[2.1.11] 
where dnp(Rp) represents the number of particles at time t, per unit volume of the reaction mixture (of the suspension), whose size ranges from Rp to Rp + dRp.
The PSD is obtained from sedimentation or by screening data on the feed to the reactor.
The integral distribution function or cumulative distribution function (CDF) is the number of particles whose size ranges from 0 to Rp per unit volume. Its expression is given by:
[2.1.12] 
The total number of particles per volume unit (i.e. the concentration) is:
[2.1.13] 
In the same way, we define a distribution function based on the mass of the particles: the mass-PSD function ω(Rp) is the mass of particles per suspension unit volume whose size ranges from Rp and Rp + dRp. The total mass of the particles per unit volume (or mass concentration) is then equal to:
[2.1.14] 
The normalized forms of the PDF are:
[2.1.15] 
The third moment with respect to normalized distribution function is the volume of unreacted particles per unit volume of slurry, which is given by:
[2.1.16] 
and the mass of the particles per suspension unit volume, which is given by:
[2.1.17] 
where ρp is the density of the particles and:
[2.1.18] 
The fractional conversion between the discharge and feed (see [VIG 11a], Chapter 7, equation [7.2.5]) is given by:
[2.1.19] 
Four different functions that have been curve-fitted to materials subject to comminution processes and then sized have been proposed [PET 91]. The integral size distribution curves are very close. It is the differential function of these distributions, ψ (Rp), however, that is required in leaching models (see Figure 2.1.3b):
– Rosin-Rammler-Bennett equation:
[2.1.20] 
[2.1.21] 
where a is the size of the particles for which 63.21% smaller than or equal to a;
– Gates-Gaudin-Schuhmann equation:
[2.1.22] 
– logarithmic equation:

– Gaudin-Meloy equation:

Figure 2.1.3 shows the integral and differential curves of these four functions. The ψ (Rp) functions are very different. The choice of the distribution function is therefore not trivial [PET 91]. Other equations have been proposed [PAP 92].
Figure 2.1.3. equation [2.1.12]equation [2.1.11]
