

Contents
Preface
CHANGES IN THE SECOND EDITION
ACKNOWLEDGMENTS
FIGURE AND TABLE ACKNOWLEDGMENTS
CHAPTER 1 A Brief History of Animals
ANIMAL ORIGINS AND THE FOSSIL RECORD
THE ANIMAL TREE
GENERAL FEATURES OF ANIMAL DESIGN AND DIVERSITY
EVOLUTION AND DEVELOPMENT: DNA AND DIVERSITY
SELECTED READINGS
CHAPTER 2 The Genetic Toolkit for Development
BEFORE THE TOOLKIT—ORGANIZERS, FIELDS, AND MORPHOGENS
THE GENETIC TOOLKIT
SHARING OF THE GENETIC TOOLKIT AMONG ANIMALS
THE TOOLKIT AND ANIMAL DESIGN
SELECTED READINGS
CHAPTER 3 Building Animals
GENE REGULATION IN METAZOANS
THE ARCHITECTURE OF GENETIC REGULATORY HIERARCHIES
THE INSECT BODY PLAN
THE VERTEBRATE BODY PLAN
REVIEW: THE GENERAL LOGIC AND MECHANISMS CONTROLLING GENE EXPRESSION IN CELLULAR FIELDS
SELECTED READINGS
CHAPTER 4 Evolution of the Toolkit
THE HISTORY OF GENE FAMILIES
CASE STUDY: EVOLUTION OF THE HOX COMPLEX
INTERPRETING THE TOOLKIT: INFERENCES ABOUT ANIMAL EVOLUTION
THE TOOLKIT AS DEVELOPMENTAL POTENTIAL
SELECTED READINGS
CHAPTER 5 Diversification of Body Plans and Body Parts
DIVERSITY OF ANTERIOR/POSTERIOR BODY ORGANIZATION WITHIN ARTHROPODS AND VERTEBRATES
MORPHOLOGICAL DIVERSITY WITHIN A CONSERVED BODY PLAN
REGULATORY EVOLUTION AND THE DIVERSIFICATION OF HOMOLOGOUS BODY PARTS
SELECTED READINGS
CHAPTER 6 The Evolution of Morphological Novelties
WHAT IS MORPHOLOGICAL NOVELTY?
NOVEL FUNCTIONS FROM OLDER MORPHOLOGICAL STRUCTURES
THE EVOLUTION OF VERTEBRATE NOVELTIES
EVOLUTION OF RADICAL BODY PLAN CHANGES
REGULATORY EVOLUTION AND THE ORIGIN OF NOVELTIES
SELECTED READINGS
CHAPTER 7 Morphological Variation and Species Divergence
EVOLUTION OF ANIMAL COLOR PATTERNS
NODAL POINTS IN REGULATORY NETWORKS AND THE EVOLUTION OF CHARACTER NUMBER AND PATTERN
QUALITATIVE AND QUANTITATIVE ASPECTS OF SKELETAL EVOLUTION IN STICKLEBACK FISH
MORE VARIATION THAN MEETS THE EYE: CRYPTIC GENETIC VARIATION AND THE POTENTIAL FOR MORPHOLOGICAL EVOLUTION
REGULATORY EVOLUTION AND SPECIES DIVERGENCE
SELECTED READINGS
CHAPTER 8 From DNA to Diversity: The Primacy of Regulatory Evolution
WHY IS REGULATORY EVOLUTION A PRIMARY FORCE IN MORPHOLOGICAL EVOLUTION?
THE FUNCTION AND EVOLUTION OF CIS-REGULATORY DNA
THE EVOLUTION OF REGULATORY DNA AND MORPHOLOGICAL DIVERSITY
SELECTED READINGS
Glossary
Index

© 2001, 2005 by Sean Carroll
BLACKWELL PUBLISHING
350 Main Street, Malden, MA 02148-5020, USA
108 Cowley Road, Oxford OX4 1JF, UK
550 Swanston Street, Carlton, Victoria 3053, Australia
The right of Sean B. Carroll, Jennifer K. Grenier, and Scott. D. Weatherbee to be identified as the Authors of this Work has been asserted in accordance with the UK Copyright, Designs, and Patents Act 1988.
All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, except as permitted by the UK Copyright, Designs, and Patents Act 1988, without the prior permission of the publisher.
First edition published 2001 by Blackwell Publishing Ltd
Second edition published 2005
Library of Congress Cataloging-in-Publication Data
Carroll, Sean B.
From DNA to diversity: molecular genetics and the evolution of animal design / Sean B.
Carroll, Jennifer K. Grenier, Scott D. Weatherbee.—2nd ed.
p.; cm.
Includes bibliographical references and index.
ISBN 1-4051-1950-0 (pbk. : alk. paper)
1. Evolutionary genetics.2. Molecular genetics. 3. Biological diversity. 4. Morphology (Animals)
[DNLM: 1. Animal Population Groups—genetics. 2. Variation (Genetics) 3. Animal Population Groups—anatomy & histology. 4. Evolution, Molecular. 5. Gene Expression.
QH 408 C319f 2005] I. Grenier, Jennifer K. II. Weatherbee, Scott D. III. Title.
QH390.C37 2005
572′38—dc22
2003027991
A catalogue record for this title is available from the British Library.
The publisher’s policy is to use permanent paper from mills that operate a sustainable forestry policy, and which has been manufactured from pulp processed using acid-free and elementary chlorine-free practices. Furthermore, the publisher ensures that the text paper and cover board used have met acceptable environmental accreditation standards.
For further information on Blackwell Publishing, visit our website:
http://www.blackwellpublishing.com
To our families
“From DNA to Diversity is written for a general audience, including undergraduates, with an interest in developmental and evolutionary biology, and it is a joy to read. Using striking examples, the authors summarize the current state of thinking on the interconnectedness between developmental genetics and evolutionary diversification.” Axel Meyer, University of Konstanz; Nature
“This book helps to fill a gap in the teaching of evolutionary theory that arose because developmental biology was not a direct participant in the evolutionary synthesis. … This is an outstanding account of the latest findings in molecular developmental biology.” James W. Valentine, Professor Emeritus, University of California, Berkeley
“The authors have done an excellent job of distilling the large and complex literature on molecular genetics that is pertinent to understanding how gene networks evolve … The writing is consistently clear, concise, and engaging.”
Gregory A. Wray, Duke University; Science
“Carroll, Weatherbee, and Grenier have produced a wonderful and exciting introduction to the field of evolutionary developmental biology … Newcomers and aficionados will find this a compelling read.”
Martin J. Cohn, University of Florida; Evolution and Development
“… this is one book that everybody should read who wants to know why ‘evo-devo’ is such a hot topic right now.”
Manfred Laubichler, Arizona State University
“From DNA to Diversity can be, and should be read by College and University students as well as scientists out of the field, who want to be informed of what is new and promising in biology.”
Jean Deutsch, Universite Phillippe et Marie Curie, Paris; BioEssays
Preface
The Earth is now populated by between 1 million and perhaps as many as 20 million animal species, which represent probably less than 1% of all animal species that have ever existed. An even more remarkable fact is that all of this diversity—aardvarks and ostriches, butterflies and pythons, dinosaurs, and earthworms—descended from a common bilaterally symmetrical ancestor that lived in Precambrian seas more than 540 million years ago. Traditionally approached through paleontology, systematics, and comparative anatomy, the story of animal evolution has, until recently, been sorely missing one huge chapter—namely, genetics.
Animals diverge from common ancestors through changes in their DNA. The major question, then, is, Which changes in DNA account for morphological diversity? The answer to this question has eluded us for the half-century since the Modern Synthesis was proposed and the structure of DNA was discovered. Although many reasons exist to explain this omission, foremost among them is that biology first had to address another central genetic mystery—that is, which genes out of the thousands in any species control morphology?
One of the most important biological discoveries of the past two decades is that most animals, no matter how divergent in form, share specific families of genes that regulate major aspects of body pattern. The discovery of this common genetic “toolkit” for animal development has had two major implications for researchers. First, it has enabled biologists to uncover widely conserved molecular, cellular, and developmental processes whose existence was concealed by previously incomparable anatomies. Second, it has focused the study of the genetic basis of animal diversity on how the number, regulation, and function of genes within the toolkit have changed over the course of animal evolution.
The genetic picture of morphological diversity presented in this book is highly influenced by the legacy of previous successes of genetic logic. The mysteries of enzyme induction in bacteria and bacteriophage life cycles were, through formal genetic logic and molecular biology, ultimately reduced to elegant genetic switches that determined the on/off state of groups of genes. This success laid the foundation for understanding the regulation of genes in different cell types of multicellular organisms and, in turn, the regulation of genes in space and over time during the development of individual organisms. Similarly, recent advances in understanding how the toolkit operates in the design of just a few model species has laid the foundation for studies of the evolution of a wide variety of animal structures and patterns.
The presentation in this book lies at the intersection of evolutionary biology with embryology and genetics. Comprehensive treatment of any of these long-established, fast-growing disciplines can be found in full textbooks dedicated to each. Because our goal is to elucidate general principles about the genetic basis of morphological change, we will focus on those genes, developmental processes, and taxa that are best known and best illustrate these principles. The book is organized into two parts. The first part (Chapters 1–3) focuses on the history of animals and on animal developmental genetics and regulatory mechanisms. We first examine some of the major trends in animal design and evolution illustrated in the fossil record and by modern forms (Chapter 1). Next, we take an inventory of the genetic toolkit for the development of model species (Chapter 2). Finally, we analyze the regulation and function of these genes in the complex hierarchies that govern animal development (Chapter 3). This crucial background knowledge of the major transitions in animal evolution and the genetic logic of animal design sets the stage for the analysis of mechanisms of morphological evolution.
The second part of the book examines the genetic mechanisms underlying the evolution of animals at different morphological levels. We take a case study approach by focusing on the best-understood examples of the evolution of the genetic toolkit, the diversity of body plans and body parts, and novel structures. In the final chapter, we discuss why and how changes in gene regulation have played a primary role in the evolution of diversity across the morphological spectrum—from small-scale differences within or between species, to the large-scale differences that distinguish higher taxa.
We have provided selected references for further reading at the end of each chapter. By no means should these citations (or this book) be taken as the primary or exclusive references on a topic. For both brevity and to circumvent questions of priority in ideas or evidence, we have avoided attributions to specific authors in the text.
One of the inspirations for our approach was Mark Ptashne’s classic A Genetic Switch, in which many of the basic physiological and molecular principles of gene regulation were illuminated by focusing on the bacteriophage λ. In the preface, Ptashne stated that “one of the charms of molecular biology is that the answers it provides to fundamental questions for the most part can be easily visualized.” Few fields in biology can rival the aesthetic appeal of the new comparative embryology. Indeed, the visualization of members of the genetic toolkit in action during the development of different species has already become a surrogate for analyzing final forms. For those who find conceptual beauty in the logic and molecular anatomy of genetic switches, the genetic switches controling animal anatomy may be even more appealing. Not only do they control the striking patterns of gene expression within developing embryos, but as we shall see, they are also key to understanding how the wonderful, but presently dwindling, diversity of animal forms has evolved.
The revision and expansion of From DNA to Diversity for this second edition is driven by advances on many fronts. Increased understanding of developmental mechanisms, systematic exploration and comparisons of animal genomes, and inquiries into new models of morphological evolution have provided a wealth of case studies from which we have selected new material. Much of the new coverage in this edition is found in the second part of the book, which has been expanded to five chapters (Chapters 4–8) from four in the first edition. Information and references have been updated throughout the book. Again, we stress that these citations are selective and that neither they nor this book should be taken as the primary or exclusive reference on a topic.
The book’s overall organization remains the same, with the first three chapters devoted to the history of animals, developmental genetics, and genetic regulatory mechanisms. The second part of the book (Chapters 4–8) examines the evolution of animals at different morphological levels. The explosion in genome sequence data has provided an enormous increase in the quantity and quality of information concerning the evolution of the genetic toolkit for animal development. Many animal genomes, including our own, have been sequenced since the publication of the first edition. Some of the major insights from genome studies have been added to Chapter 4.
The growth of evolutionary developmental biology has provided new insights into the diversification of specific body plans and the origins of animal novelties. Chapters 5 and 6 have been revised and expanded to incorporate new findings ranging from mechanisms of segmentation in spiders, to the evolution of the cephalopod body plan, and the origin of the turtle shell.
There has also been an increasing focus on models of variation within species and of divergence of traits. Some of the simplest models of phenotypic variation and evolution involve the color patterns of mammals, birds, and insects. In several cases, the identity of genetic differences responsible for variation between populations is now known. We have added a new chapter (Chapter 7) that focuses on models of variation and divergence among closely related species.
We thank all of our colleagues who have shared their discoveries with us and allowed us to use their illustrations in this book. This edition would not have been possible without the constructive input of reviewers and users of the first edition; we thank you for your thoughtful comments and suggestions. We also thank Jamie Carroll for design of the cover, preparation of the text, and coordination of the editing and permissions process; Leanne Olds for additional artwork; Steve Paddock for help with images; and Nancy Whilton for her unflagging support for this book from the very beginning. We thank the many current and past members of the Carroll laboratory for their insights and hard work in developmental and evolutionary biology, without which this endeavor would not have been possible, and Lee Niswander for her support and encouragement. The authors’ work has been supported by the Howard Hughes Medical Institute, National Science Foundation, National Institutes of Health, Sloan Kettering Institute, Shaw Scientist’s Program of the Milwaukee Foundation, Human Frontiers Science Program, European Molecular Biology Organization, and the University of Wisconsin.
The authors and publisher gratefully acknowledge the permission granted to reproduce the copyright material in this book.
Every effort has been made to trace copyright holders and to obtain their permission for the use of copyright material. The publisher apologizes for any errors or omissions, and would be grateful to be notified of any corrections that should be incorporated in future reprints or editions of this book.

... an understanding of regulation must lie at the center of any rapprochement between molecular and evolutionary biology; for a synthesis of the two biologies will surely take place, if it occurs at all, on the common field of development.
—Stephen Jay Gould
Ontogeny and Phylogeny (1977)
The central focus of this book is to identify the genetic mechanisms underlying the evolution of animal design, particularly with regard to the patterning of animal body plans and body parts. To approach this mystery, new discoveries and ideas from developmental genetics must be integrated into the larger framework of the evolutionary history of animal life. This history is reconstructed from many fields of study—in particular, paleontology, systematics, and comparative biology. In this chapter, we present a brief overview of animal evolution from these three perspectives. This discussion provides a historical foundation for the consideration of the mechanistic questions that are addressed in subsequent chapters.
First, we discuss the origin of animals and the radiation of the major animal phyla based on evidence gleaned from the fossil record. Most living phyla have ancient origins, and the fundamental differences between them evolved long ago. Two milestones in early animal history that are of special interest are the evolution of bilaterally symmetrical animals and the explosive radiation of these forms in the Cambrian period more than 500 million years ago.
Second, we examine the phylogenetic relationships among animals. Understanding the direction of evolutionary change in morphological, developmental, or genetic traits and the ability to make inferences about animal ancestors requires knowledge of the structure of the animal evolutionary tree. While traditionally based upon morphological comparisons, new phylogenies based on DNA and protein sequences have revealed unexpected relationships among anatomically disparate animals, refuting long-held notions about which phyla are more closely related.
Third, we consider the comparative anatomy of selected phyla with the aim of identifying some of the major trends in the evolutionary diversification of individual phyla. In particular, we focus on the modular organization of the body plans and body parts of larger animals—the vertebrates, arthropods, and annelids. Much of the large-scale morphological diversity within these phyla (for example, between different classes) involves differences in the number and pattern of modular elements (segments, appendages, and so on). The recognition of the modular organization of these animals is an important conceptual link to understanding the genetic logic controlling their development and the mechanisms underlying the evolution of diversity.
The fossil record is our primary window into the history of life. It provides many kinds of information that cannot be inferred from living animals. Fossils give us pictures of extinct forms that may be ancestors of modern animals, provide minimal estimates of the time of origin or divergence of particular groups, reveal episodes of extinctions and radiations, and, in favorable circumstances, offer detailed accounts of the evolution of important structures.
The search for the origins of modern animals begins with an assessment of the Cambrian fossil record. It has been known since before Darwin’s time that animal diversity increased dramatically during this period, which spans an age from roughly 545 to 490 million years ago (Ma). Molluscs, arthropods, annelids, chordates, echinoderms, and representatives of most other modern phyla make their first appearance in Cambrian fossil deposits (Fig. 1.1). The emergence of large, complex animal forms and their radiation over a 10 to 25 million year interval in the Early–Middle Cambrian is often referred to as the “Cambrian Explosion.”
The appearance of these animals in the Cambrian fossil record gives us only a minimum estimate of their time of origin. The crucial question about the Cambrian Explosion is whether it marks the origin of animals or the origin of modern phyla. Did most phyla first arise in this short period, or did they predate their preservation in the Cambrian fossil record? Although the Precambrian animal fossil record is relatively scarce, several kinds of fossil evidence indicate that the origins of most modern phyla predate the Cambrian. First, the fossil record of some modern groups clearly begins before this period. For example, body fossils of both cnidarians and sponges predate the Cambrian (Fig. 1.2). Both of these groups are diploblastic animals, composed of two tissue layers. The cnidarians have a radically symmetrical body design that distinguishes them from sponges and from a much larger number of modern phyla that are triploblastic—that is, composed of three tissue layers—and have bilaterally symmetrical body designs (the Bilateria). Second, Precambrian deposits contain evidence in the form of trace fossils, the record of the meanderings and burrowings of animals in sediments, which indicate the existence of some bilaterian forms (Figs 1.2 & 1.3d) well before the Cambrian Explosion. A third piece of potential evidence for earlier animal origins is the Ediacaran fauna (575–544 Ma), named for the Australian locale in which they were first discovered.
The biological interpretation of Ediacaran fossils and their relationships, if any, to modern animals remains controversial. Several distinct body plans have been identified, including radially symmetrical types and a number of frond-like and tube-like forms (Fig. 1.3). None of these bear any clear-cut similarity to modern animals, so they have been difficult to place on the tree of animal evolution. Some of the Ediacaran fossils could represent diploblastic forms related to cnidarians or sponges. Others could be primitive bilaterians that possess some, but not all, features of modern bilaterians.
The difficulties in placing Ediacarans in the scheme of animal evolution have led to the proposal that they represent an extinct experiment in multicellular life. On the other hand, perhaps their lack of resemblance to modern groups is exactly what should be expected of primitive animals. It is possible that the Ediacaran fauna include both extinct types of diploblastic animals and primitive ancestors of modern bilaterians. The fossil record indicates that some Ediacaran forms persisted into the Cambrian, but then died out as bilaterians, sponges, cnidarians, and ctenophores flourished.
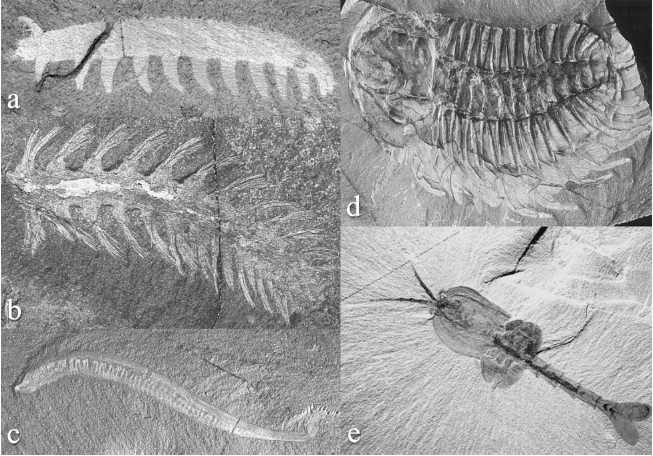
Figure 1.1 Cambrian animal fossils Representatives of many modern phyla are found in Cambrian deposits and are made up of repeating units. (a) Aysheaia pedunculata, an onychophoran; (b) Burgessochaeta setigera, a polychaete annelid; (c) Pikaia gracilens, a chordate; (d) Olenoides serratus, a trilobitomorph arthropod; (e) Waptia fieldensis, a crustacean-type arthropod.
Source: Photographs from Briggs DEG, Erwin DH, Collier FJ. Fossils of the Burgess shale. Washington, DC: Smithsonian Institution Press, 1994; reprinted by permission from the Smithsonian Institution Press.
Given the uncertainty of the relationship of the Ediacarans to modern phyla and the paucity of body fossils prior to the Cambrian, it is difficult to pinpoint the origins of modern animals based on the fossil evidence. Consequently, biologists have turned to other methods to try to identify when major animal groups diverged. Using the evolution of protein and ribosomal RNA sequences between species to calibrate molecular clocks, estimates of the time of divergence of most animal phyla have been made that range from approximately 650 Ma to more than 1000 Ma. While these estimates remain controversial, even the most conservative estimate suggests a period of more than 100 million years before the beginning of the Cambrian in which most bilaterian phyla had arisen but led a paleontologically cryptic existence.
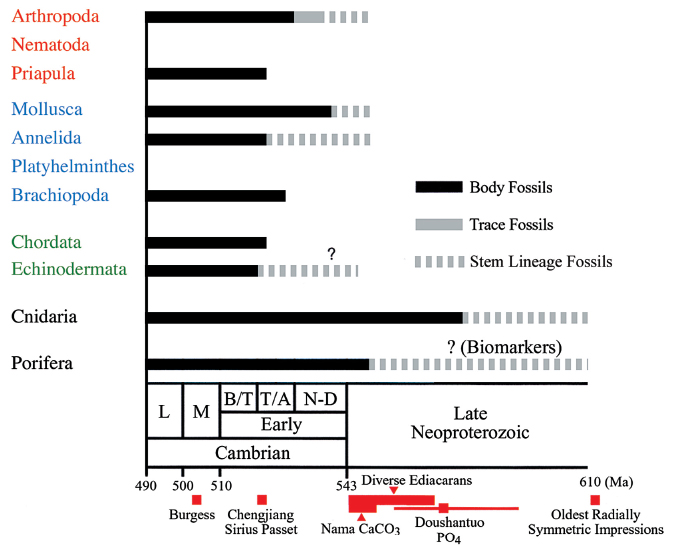
Figure 1.2 The early fossil record of animals The appearance of various animal phyla in the fossil record are indicated, relative to the Cambrian and Proterozoic periods. The ages of fossils from particular localities are shown in red at the bottom. Note that the cnidarian and poriferan records clearly predate the Cambrian. Other phyla first appear in the Cambrian, although early members may exist that predate the Cambrian by a considerable period. L, Late; M, Middle; B/T, Botomian plus Toyonian; T/A, Tommotian plus Atdabanian; N-D, Nemakit-Daldynian.
Source: Adapted from Knoll AH, Carroll SB. Science 1999;284:2129–2137.
It is widely believed that primitive bilaterians may have been very small and their size limited by atmospheric and oceanic oxygen levels. This fact would help to explain their slim fossil record before the Cambrian (Fig. 1.2). In the last few years, evidence has also been gathered that suggests a possible mass extinction at the boundary between the Proterozoic and Cambrian. Whatever the cause of such an event, it may have hastened the extinction of Ediacaran forms and opened up the ecological opportunity for bilaterians to radiate. Environmental and ecological changes may have removed constraints on bilaterians, permitting the evolution of larger animals. In addition, competitive interactions among bilaterians may have facilitated the evolution of skeletonized taxa, more sophisticated predatory and defense behaviors, and the variety of anatomical innovations that unfolded in the Cambrian.
Figure 1.3 Pre-Cambrian animal fossils and traces (a) Ediacaria, a radially symmetrical form from deposits in Australia. (b) Calcified fossils in limestone from Namibia. (c) Pteridinium, a frond-like ediacaran fossil form built of repeating units. (d) Trace fossils made in sediments by bilaterian animals.
Source: Knoll AH, Carroll SB. Science 1999;284:2129–2137.

There are about 35 living animal phyla. To understand the origin and evolution of any feature found in one or more of these groups, it is necessary to have a picture of the phylogenetic relationships among animals. Ideally, the fossil record would present a complete, ordered, unambiguous picture of the branching pattern of the animal tree. Unfortunately, it does not. As the divergence of most bilaterian phyla appears to have predated the emergence of recognizable members of modern phyla in the fossil record, we must make our inferences from later, more derived forms.
Constructing an accurate picture of metazoan relationships has been challenging, and many alternative schemes of animal phylogeny have been proposed and scrutinized over recent decades and continue to be evaluated. Most approaches have relied on anatomical and embryological comparisons. In general, phylogenies are determined according to shared characters that are presumed to be derived and therefore reflect a close relationship. For example, all animal phyla are thought to be more closely related to each other than to any other nonanimal phylum, because of similarities in animal multicellularity, cell structure and morphology, and cell signaling. Members of the most closely related protist group, the choanoflagellates, share a similar cell architecture with sponges but are not multicellular. What is most difficult to determine is whether apparent similarities between animals (for example, segmentation in arthropods and annelids) are due to common ancestry, are superficial, or evolved independently. Also, different tree topologies can emerge when different characters are used or when the same characters are weighted differently.
One way to circumvent the reliance on morphological comparisons is to use molecular genetic characters to construct animal phylogenies. As taxa diverge, the sequences of DNA, RNA, and protein molecules diverge as well; the relative degree of divergence can therefore be used to infer phylogenetic relationships. In addition, the presence or absence of particular genes, or the linkage of a group of genes on chromosomes, can be used to construct phylogenetic trees. New methods based on molecular sequences have been combined with morphology-based approaches to both prune and strengthen the animal tree.
We now recognize shared morphological, developmental, and genetic traits that suggest that the Bilateria can be organized into three great clades (a set of species descended from a common ancestor) (Fig. 1.4):
Figure 1.4 Metazoan phylogeny The current picture of metazoan phylogeny showing representatives of three major bilaterians clades—the deuterostomes, the Lophotrochozoa, and the arthropod + onychophora + priapulid clade.
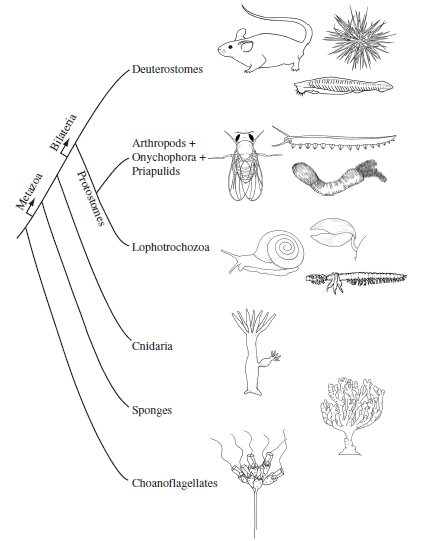
Within these great clades, the branching order has been less well resolved, such that it is unclear which phyla are more closely related. It is worth noting that the recent assignment of arthropods and annelids to two different protostome clades and the assignment of pseudocoelomate phyla among different clades are major changes from previous portraits of the animal tree. The phylogenetic placement of the nematodes, including the model organism Caenorhabditis elegans, remains controversial, because their rapid molecular clock complicates analysis. Some phylogenies place the nematodes close to the arthropod + onychophora + priapulid clade and others more basally near the common ancestor of all bilaterian phyla.
The anatomical and developmental features of the Bilateria are very distinct from those of the basal metazoans (cnidara, ctenophores, and porifera). The evolutionary links between basal metazoans and the bilaterians are difficult to perceive. Indeed, as we will see in Chapter 4, major differences exist between the genetic toolkit of these two groups, and the differences are much more substantial than those between most bilaterians. Because of the long divergence time since the radiation of these groups, the phylogenetic relationships between cnidarians, sponges, and ctenophores and the last common ancestor of the Bilateria are uncertain. Many extinct animal lineages, as yet unknown from the fossil record, may have branched off of the metazoan tree between the last common ancestor of all animals and of the Bilateria (Fig. 1.4).
The gaps in the fossil record; the great differences in anatomy, development, and genome content between radially symmetrical animals and bilaterians; and the cryptic early history of bilaterians, make inferences about the morphological transformations involved in the origin of animal body plans very speculative. Paleontologists have introduced the concept of disparity to refer to differences among body plans and use the term diversity to refer to the number of species within a group. The genetic and developmental bases of the morphological diversification of a particular body plan within a phylum are far more accessible than is the origin of different body plans. Therefore, we will focus primarily on evolutionary trends within a few select phyla, such as the arthropods and chordates, making the implicit assumption that the same sort of genetic mechanisms involved in the evolution of large-scale morphological diversity within phyla also gave rise to fundamental differences in body plans.
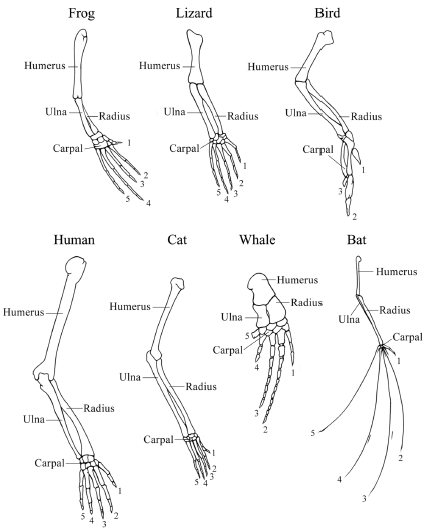
One of the most outstanding features of animal design, particularly of larger bilaterians, is their construction from repeating structures (or modules). The segments of arthropods and annelids and the vertebrae (and associated processes) of vertebrates are the basic units of body plan organization in these phyla (Fig. 1.5a–c). Similarly, many body parts such as the insect wing (Fig. 1.5d) and the tetrapod hand (Fig. 1.5e) are composed of repeated structures.
An important trend in the morphological evolution of animals has been the individualization of modular elements. For example, among the arthropods, we observe a large number of different segment types in crustaceans and insects. This diversity far exceeds that found in the onychophora, a phylum closely related to the arthropods. Thus the evolution of the onychophoran/arthropod clade has been marked by increased diversity of segment types from the more uniform patterns found in earlier forms. Similarly, in some mammals, teeth are differentiated into molars, premolars, canines, and incisors, whereas in the ancestral condition exhibited by most reptiles, the teeth are of uniform shape. Because the diversification of the number, morphology, and function of these repeated units characterizes many of the large-scale differences that distinguish related taxa, understanding how repeated structures form and become individualized is a prerequisite for understanding the developmental basis of large-scale morphological evolution.
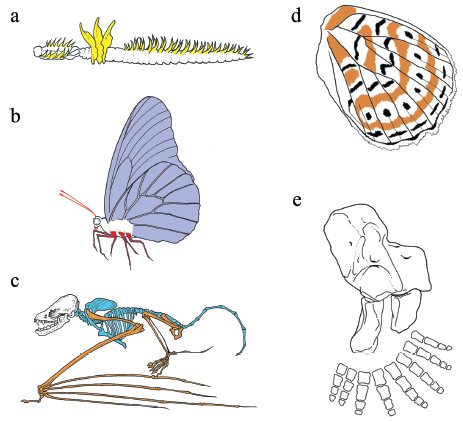
Figure 1.5 The modularity of body plans and body parts The body plans of many major phyla, including the annelids (a), arthropods (b), and chordates (c), are composed of many repeating parts. Some of these parts are similar or identical in appearance to other parts; others are individuated. Sets of serially homologous structures are shaded a unique color. Body parts, such as a butterfly wing (d), or a fossil tetrapod limb from the amphibian fossil Acanthostega (e), are also composed of repeating structures or patterns, some of which are differentiated from others. For example, Acanthostega has eight digits, but like its modern descendants, only five distinct types of digits can be distinguished.
Source: Parts a–c from Weatherbee SD, Carroll SB. Selector genes and limb identity in arthropods and vertebrates. Cell 1999; 97: 283–286; part e from Michael Coates.
The modular organization of animal bodies and body parts has long been recognized by comparative biologists. William Bateson, in his classic treatise Materials for the Study of Variation (1894), identified several kinds of organization found among animals. More importantly, he was the first to bring a Darwinian perspective to the question of how different body patterns may have evolved. Bateson focused particularly on the repetition of parts, cataloguing a large number of rare, but naturally occurring, variants that differed from the norms within various species with regard to either the number or individualization of characters. He suggested that these variations within species could provide insight into the evolution of the large-scale morphological discontinuities between species. For example, variations in the number of body segments within onychophora and centipede species, and of vertebrae in humans and pythons, suggested to Bateson that such discontinuities arose at some frequency in populations and therefore represented plausible steps in the morphological diversification of species.
The question of whether evolution may progress in large, discrete steps remains controversial (we will address this issue in Chapter 8). Nevertheless, these sorts of variants and the organizational concepts espoused by Bateson have been enormously helpful in understanding the genetics and developmental logic underlying the modularity of animal design. In fact, they led to the discovery of genes that play key roles in morphological evolution, albeit not in the fashion Bateson first imagined.
Four fundamental kinds of large-scale, evolutionary differences in morphology are most prevalent in modularly organized animals and are the most significant in terms of adaptation:
Figure 1.6 Meristic differences among arthropods and among vertebrates Among arthropods such as this trilobite (a), crustacean (b), centipede (c), and insect (d), the number of body segments differs, as does the diversity of segment morphology. Among vertebrates, the number of vertebrae and associated processes differs considerably between a fish (e), frog (f), python (g), and chimpanzee (h).
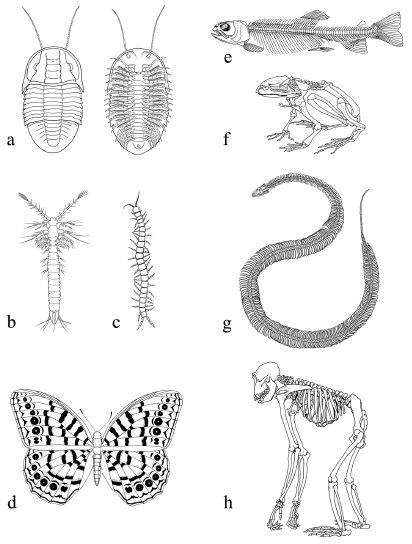
Figure 1.7 The diversification of homologous parts All vertebrate forelimbs are homologous structures whose anatomy has undergone considerable diversification in the evolution and adaptation of these various vertebrate lineages. Not to scale.
Source: Redrawn from Ridley M. Evolution, 2nd edn. Malden, MA: Blackwell Science, 1996.
Considering that modularly organized animals are among the most diverse groups (in terms of both the number and morphology of species), could there be a correlation between body design and evolutionary diversity? One possible explanation for this relationship is that modular organization allows one part of the animal to change without necessarily affecting other parts. The evolution of genetic mechanisms that control the individualization of parts would allow for the uncoupling of developmental processes in one part of the body from the developmental processes in another part of the body. In this fashion, for example, vertebrate forelimbs can evolve into wings while hindlimbs remain walking legs. Dissociation of the forelimb and hindlimb developmental programs allows further modifications to occur selectively in either structure, such as the development of feathers in the forelimb of birds and scales in the hindlimb.
To understand the major trends in animal diversity and the various kinds of morphological evolution, we must first understand how animal form is generated. Morphology is the product of development, the process through which a single fertilized egg cell gives rise to an entire organism. The physical basis of animal diversity has been viewed since Darwin’s time as the outcome of development. Until very recently, however, the developmental principles underlying animal design remained unknown. Although experimental embryologists of the late 1800s and the first half of the 1900s had identified many fascinating phenomena concerning the organization of embryos and the formation of particular structures, the mechanisms responsible for these properties were beyond their reach.
With better understanding of the nature of genes and the process of gene regulation, development has been increasingly viewed as a process orchestrated by the products of genes. Thus the puzzles of embryology, such as how cells come to know their position and identity within a developing animal, have become rephrased in genetic terms. Given that the DNA of (most) all cells in an animal is identical, how do different cells acquire the unique morphologies and functional properties required in the diverse organs and tissues of the body? We now understand that this process occurs through the selective expression of distinct subsets of the many thousands of genes in any animal’s genome in different cells. How genes are turned on and off in different cells over the course of animal development is an exquisitely orchestrated regulatory program whose features are only now coming into detailed view.
If morphological diversity is all about development, and development results from genetic regulatory programs, then is the evolution of diversity directly related to the evolution of genetic regulatory programs? Simply put, yes. But to understand how diversity evolves, we must first understand the genetic regulatory mechanisms that operate in development. In other words, what is the genetic toolkit of development and how does it operate to build animals? In the next two chapters, we will examine some of the general features of the genetic and regulatory logic of animal development.
Chen JY, Oliveri P, Gao F, et al. Precambrian animal life: probable developmental and adult cnidarian forms from Southwest China. Dev Biol 2002; 248, 182–196.
Chen JY, Oliveri P, Li CW, et al. Precambrian animal diversity: putative phosphatized embryos from the Doushantuo Formation of China. Proc Natl Acad Sci USA 2000; 97: 4457–4462.
Conway MS. The fossil record and the early evolution of the Metazoa. Nature 1993; 361: 219–225.
_____. The crucible of creation: the Burgess shale and the rise of animals. Oxford, UK: Oxford University Press, 1998.
Fortey R. The Cambrian evolutionary “explosion”: decoupling cladogenesis from morphological disparity. Biol J Linnean Soc 1996; 5713–5133.
Glaessner MF. The dawn of animal life: a biohistorical study. Cambridge, UK: Cambridge University Press, 1984.
Gould SJ. Wonderful life: the Burgess shale and the nature of history. New York: Norton, 1989.
Knoll AH, Carroll SB. Early animal evolution: emerging views from comparative biology and geology. Science 1999; 284: 2129–2137.
Valentine JW, Jablonski D, Erwin DH. Fossils, molecules and embryos: new perspectives on the Cambrian explosion. Development 1999; 126: 851–859.
Ayala FJ, Rzhetsky A, Ayala FJ. Origin of the metazoan phyla: molecular clocks confirm paleontological estimates. Proc Natl Acad Sci USA 1998; 95: 606–611.
Wray G, Levinton J, Shapiro L. Molecular evidence for deep precambrian divergences among metazoan phyla. Science 1996; 274: 568–573.
Adoutte A, Balavoine G, Lartillot N, de Rosa R. Animal evolution: the end of the intermediate taxa? Trend Genet 1999; 15: 104–108.
Aguinaldo AM. Evidence for a clade of nematodes, arthropods, and other moulting animals. Nature 1997; 387: 489–493.
Blair JE, Ikeo K, Gojobori T, Hedges SB. The evolutionary position of nematodes. BMC Genomics 2002; 2: 7.
King N, Carroll SB. A receptor tyrosine kinase from choanoflagellates: molecular insights into early animal evolution. Proc Natl Acad Sci USA 2001; 98: 15032–15037.
Maley LE, Marshall CR. The coming of age of molecular systematics. Science 1998; 279: 505–506.
Snell EA, Furlong RF, Holland PW. Hsp70 sequences indicate that choanoflagellates are closely related to animals. Curr Biol 2001; 11: 967–970.
Bateson W. Materials for the study of variation. London: Macmillan, 1894.
Gilbert S, Opitz J, Raff R. Resynthesizing evolutionary and developmental biology. Dev Biol 1996; 173: 357–372.
Raff R. The shape of life. Chicago: University of Chicago Press, 1996.
Wagner GP. Homologues, natural kinds and the evolution of modularity. Am Zoologist 1996; 36: 36–43.
_____. The origin of morphological characters and the biological basis of homology. Evolution 1989; 43: 1157–1171.

The only way in which we may hope to get at the truth is by the organization of systematic experiments in breeding, a class of research that calls perhaps for more patience and more resources than any other form of biological inquiry. Sooner or later such investigation will be undertaken and then we shall begin to know.
— W. Bateson Material for the Study of Variation (1894)
… if the mystery that surrounds embryology is ever to come within our comprehension, we must … have recourse to other means than description of the passing show.
— T.H. Morgan Experimental Embryology (1927)
The foremost challenge for embryology has been to identify the genes and proteins that control the development of animals from an egg into an adult. Early embryologists discovered that localized regions of embryos and tissues possess properties that have long-range effects on the formation and patterning of the primary body axes and appendages. Based on these discoveries, they postulated the existence of substances responsible for these activities. However, the search for such molecules proved fruitless until the relatively recent advent of genetic and molecular biological technologies. The most successful approach to understanding normal development has involved the isolation of single gene mutations that have discrete and often large-scale effects on body pattern.
In this chapter, we take an inventory of the essential genetic toolkit for animal development. We concentrate on genes first discovered in insects, where systematic screens for developmental genes were pioneered. Importantly, however, it turns out that related genes are present in many other animals. We describe how members of the genetic toolkit were identified and what kinds of gene products they encode. In addition, we illustrate the general correlation between these genes’ patterns of expression with the development of the morphological features they affect. Finally, we briefly survey their distribution and function in other animals.
Only a small fraction of all genes in any given animal constitute the toolkit that is devoted to the formation and patterning of the body plan and body parts. Two classes of gene products with the most global effects on development are of special interest: families of proteins called transcription factors that regulate the expression of many other genes during development, and members of signaling pathways that mediate short- and long-range interactions between cells. The expression of specific transcription factors and signaling proteins marks the location of many classically defined regions within the embryo. These proteins control the formation, identity, and patterning of most major features of animal design and diversity.
Long before any genes or proteins affecting animal development were characterized, embryologists sought to identify the basic principles governing animal design. In their search, they focused on the large-scale organization of the primary body axes, the differentiation of various germ layers (ectoderm, mesoderm, and endoderm), and the polarity of structures such as appendages and insect segments. By manipulating embryos and embryonic tissues, primarily by transplantation and ablation, researchers discovered many important properties of developing embryos and tissues. Much of the fascination of embryology stems from the remarkable activities of discrete regions within developing embryos in organizing the formation of body axes and body parts. Furthermore, these classical concepts of embryonic organization present a very useful framework for considering how that organization can change during evolution. We will briefly review some of these experiments and ideas before addressing their genetic and molecular manifestations.
The first demonstration of organizers—regions of embryos or tissues that have long-range effects on the fate of surrounding tissues—was achieved by Mangold and Spemann in 1924. They transplanted the lip of the blastopore, the invagination where mesoderm and endoderm move inside the amphibian embryo, of a newt gastrula into another newt embryo and found that the transplanted tissue could induce a second complete body axis (Fig. 2.1a). The additional embryo induced was partly derived from the transplanted graft and partly derived from the host. The equivalent of the “Spemann organizer” in amphibians has been found in chick and mouse embryos, and it is now recognized to be a structure characteristic of all chordate embryos.
Other organizers with long-range effects on surrounding tissues have been identified in the developing vertebrate limb bud. Transplantation of a discrete patch of posterior tissue to an ectopic anterior site induces the formation of limb structures (digits, tendons, muscles) with mirror-image polarity to the normal anteroposterior order (Fig. 2.1b). By contrast, transplantation or removal of anterior tissue has no effect on limb development, suggesting that this posterior region of the limb bud, dubbed the zone of polarizing activity (ZPA), organizes anteroposterior (that is, the thumb-to-pinkie axis) polarity and limb formation.
Another organizer operates from the most distal tip of the limb bud, the apical ectodermal ridgeFig. 2.1b