
Contents
Foreword
Dedication
Chapter 1 Properties of materials - tensile properties
Mechanical properties
Chapter 2 Toughness, elastic/plastic behavior and hardness
Fracture toughness
Elastic and plastic behavior
Determining mechanical properties
Abrasion and wear resistance
Chapter 3 Physical properties of materials
Thermal properties
Electrical properties
Color and appearance
Chapter 4 Gypsum materials
Gypsum materials
Factors in setting
Physical properties
Comparative properties
Chapter 5 Dental waxes
Introduction
Wax properties
Chapter 6 Inelastic impression materials
Factors in impression-making
Inelastic impression materials
Chapter 7 Elastic impression materials
Alginate (irreversible) hydrocolloids
Agar–agar (reversible) hydrocolloid
Polysulfide rubber
Condensation-cured silicones
Addition-cured silicones (polysiloxanes)
Polyether impression materials
Mixing
Properties of elastic impression materials
Tray adhesives
Chapter 8 Denture base materials
Polymerization reactions
Polymer properties
Denture base polymers
Problems with denture bases
Denture teeth
Chapter 9 Investments and casting
Casting and investments
Gypsum-bonded investment
High-temperature investments
Chapter 10 Precious metal alloys
Gold and noble metals
Gold alloys
Heat treatment of gold
Considerations in casting
Problems with castings
Chapter 11 Base metal alloys
Cast chromium alloys
Physical/mechanical properties
Titanium and titanium alloys
Orthodontic wires
Chapter 12 Dental cements
Cement categories
Provisional luting agents
Water-based dental cements for permanent luting
Chapter 13 Resin-modified and resin cements
Zinc oxide cements
Hybrid ionomer cements
Resin cements
Chapter 14 Cavity varnishes, liners and bases
Cavity varnishes
Cavity liners
Low-strength bases
High-strength bases
Chapter 15 Dental amalgam
Dental amalgam alloys
Setting reaction
Physical properties
Manipulation and handling properties
Corrosion
Chapter 16 Adhesive dentistry
Principles
Adhesion and cohesion
Dental adhesion
Chapter 17 Bonding to dentin
Dentin bonding
Bonded dentin
Chapter 18 Composite restorative resins
Dental composites
Compomers
Chapter 19 Dental porcelain
Manufacture
Composition
Dental porcelains
Structure
Chapter 20 Manipulation and properties of porcelain
Manipulation
Firing (sintering)
Physical properties
Chapter 21 Advanced ceramic systems
Porcelain strengthening
Hot-pressed ceramics
Chapter 22 Porcelain bonding alloys
PFM restorations
Ceramic–noble metal systems
Ceramic–base metal systems
Casting of PFM copings
Chapter 23 Restorative materials in endodontics
Canal instrumentation
Canal irrigation
Sealer cements
Canal obturation
Endodontic leakage
Chapter 24 Orthodontic materials
Intra-oral appliances
Extra-oral appliances and jaw orthopedics
Removable orthodontic appliances
Fixed orthodontic appliances
Chapter 25 Grinding, polishing and finishing
Material removal
Coolant/lubricant effects
Finishing and polishing regimens
Chapter 26 Adverse effects of dental biomaterials
Categories of biomaterials
Toxicity and carcinogenicity
Hypersensitivity and allergic reactions
Chapter 27 Enamel, dentin and cementum
Dental enamel
Dentin
Cementum
Fluoridation of enamel
Strontium
Chapter 28 Bone
Mineral composition
Structure
Development and aging
Dental implants and bone
Glossary
Index

This edition first published 2010
© 2010 J. Anthony von Fraunhofer
Blackwell Publishing was acquired by John Wiley & Sons in February 2007. Blackwell’s publishing programme has been merged with Wiley’s global Scientific, Technical, and Medical business to form Wiley-Blackwell.
Registered office
John Wiley & Sons Ltd, The Atrium, Southern Gate, Chichester, West Sussex, PO19 8SQ, United Kingdom
Editorial offices
9600 Garsington Road, Oxford, OX4 2DQ, United Kingdom
2121 State Avenue, Ames, Iowa 50014-8300, USA
For details of our global editorial offices, for customer services and for information about how to apply for permission to reuse the copyright material in this book please see our website at www.wiley.com/wiley-blackwell.
The right of the author to be identified as the author of this work has been asserted in accordance with the Copyright, Designs and Patents Act 1988.
All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, except as permitted by the UK Copyright, Designs and Patents Act 1988, without the prior permission of the publisher.
Wiley also publishes its books in a variety of electronic formats. Some content that appears in print may not be available in electronic books.
Designations used by companies to distinguish their products are often claimed as trademarks. All brand names and product names used in this book are trade names, service marks, trademarks or registered trademarks of their respective owners. The publisher is not associated with any product or vendor mentioned in this book. This publication is designed to provide accurate and authoritative information in regard to the subject matter covered. It is sold on the understanding that the publisher is not engaged in rendering professional services. If professional advice or other expert assistance is required, the services of a competent professional should be sought.
Library of Congress Cataloging-in-Publication Data
Von Fraunhofer, J. A. (Joseph Anthony)
Dental materials at a glance / J. Anthony von Fraunhofer.
p. ; cm. – (At a glance series)
Includes index.
ISBN 978-0-8138-1614-2 (pbk. : alk. paper)
1. Dental materials. I. Title. II. Series: At a glance series (Oxford, England).
[DNLM: 1. Dental Materials–Handbooks. WU 49 V945d 2010]
RK652.5.V66 2010
617.6′95–dc22
2009009870
A catalogue record for this book is available from the British Library.
1 2010
If you are reading this, then it is a fair guess that you are doing so because dental materials (a.k.a. dental biomaterials science) is a requirement of a much larger field of study such as dentistry, dental hygiene or possibly one of the many post-graduate specialties within dentistry. That being the case, as the reader, you want a concise and up-to-date compendium of the information that you need to pass state and national boards as well as course and similar examinations as expeditiously as possible. This book is designed for that purpose.
Every effort has been made to provide good coverage of every important and significant area of dental materials science. Nevertheless, it is important that the reader understands the coverage provided here cannot hope to rival that of the much larger standard texts in the field. Further, the reader is encouraged to consult these texts, indicated below, when there is a need for more detailed coverage. While the underlying science and/or mechanisms have been addressed in the majority of topics, the reader is likewise encouraged to consult the standard texts when an in-depth explanation of certain topics is needed.
Finally, this book is not intended to replace lectures and formal course work but rather to function as a concise guide and expanded revision notes to the large and somewhat complex field of dental materials science. Further, mention is made in this book of Standards and Specifications. These are references to ADA/ANSI and ISO specifications which are readily available and, accordingly, details are not stated here.
On a personal note, I should like to express my appreciation of my wife Susan for her patience, support and forbearance while I wandered around muttering to myself during the writing of this book. I should also like to thank Dr Les Gartner, Dr Greg Kurtzman, Dr Tom Glass, Dr Stan Conrad, Dr Nicole Brummer and Dr Cornelius (Pam) Pameijer for reading and commenting upon various chapters and, especially, John J. Kim, DDS, for taking the time and trouble to read everything. The advice, comments and suggestions from my friends and colleagues have been invaluable.
J. A. von Fraunhofer
Baltimore, MD
June, 2009
Applied Dental Materials, 9th edn. J. F. McCabe and A. W. G. Wells, Blackwell, Oxford (2008).
Craig’s Restorative Dental Materials, 12th edn. J. M. Powers and R. L. Sakaguchi (editors), Mosby-Elsevier, St. Louis (2006).
Phillips’ Science of Dental Materials, 11th edn. K. J. Anusavice (editor), Saunders-Elsevier Science, St. Louis (2003).
Dedication
This book is dedicated to Dental Students, for they are the future of dentistry, and to the Faculty of Dental Schools, because their expertise and dedication make it all possible.
‘Every tooth in a man’s head is more valuable than a diamond.’
Miguel de Cervantes, Don Quixote (1605)
Figure 1.1 Applied forces and specimen deformations.

Figure 1.2 Load vs. stress for feet.

Figure 1.3 The stress–strain curve of a non-ferrous metal.

Figure 1.4 Stress–strain curves for a brittle, an elastic and a ductile material.

Figure 1.5 Elastic and plastic regions of a stress–strain curve.

Table 1.1 Desirable properties of dental materials.
| Biocompatibility |
| Absence of toxicity |
| Esthetic appearance |
| Strength and durability |
| Low solubility |
| Ease of manipulation |
| Long shelf life |
| Simple laboratory processing |
| Long working time |
| Rapid/snap set |
Table 1.2 Typical mechanical properties of dental biomaterials.
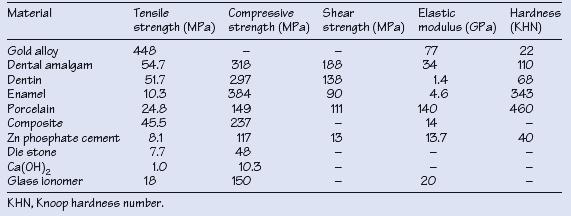
Dental biomaterials are used in laboratory procedures and for the restoration and replacement of teeth and bone. Material selection must consider function, properties and associated risks, and all dental biomaterials must satisfy certain criteria (Table 1.1).
Mechanical properties are important since teeth and restorations must resist biting and chewing (masticatory) forces. Typical properties are given in Table 1.2.
Biting forces vary with patient age and dentition, decreasing for restored teeth and when a bridge, removable partial denture (RPD) or complete denture is present. Effects vary with the type of applied force and its magnitude. Types of applied force, and the resulting deformations, are shown in Figure 1.1.
Stress, σ: force per unit cross-sectional area. Strength is the stress that causes failure. Ultimate strength is the maximum stress sustained before failure.
Stress, the applied force and the area over which it operates, determines the effect of the applied load. For example, a chewing force of 72 kg (10 N) spread over a quadrant 4 cm2 in area exerts a stress of 18 kg/cm2 (1.76 MPa). However, the same force on a restoration high spot or a 1-mm2 hard food fragment produces a stress of 7200 kg/cm2 (706 MPa), a 400-fold increase in loading. This stress effect is one reason that occlusal balancing is essential in restorative dentistry. A more graphic example of the difference between applied force and stress is shown in Figure 1.2. This example also clearly indicates why it is more painful when a woman wearing high heels steps on you than when a man does!
Proportional limit is the maximum stress that the material can sustain without deviation from a linear stress–strain proportionality.
Elastic limit is the maximum stress that can be applied without permanent deformation.
Yield strength, σy is the stress at which there is a specified deviation from proportionality of stress to strain. It is usually 0.1, 0.2 or 0.5% of the permanent strain.
Strain, ε : ratio of deformation to original length ΔL/L. Strain measures deformation at failure.
Ductility: percentage elongation, i.e. ΔL/L × 100%.
Ductile materials exhibit greater percentage elongations than brittle materials and can withstand greater deformation before fracture.
Burnishing index: ability of a material to be worked in the mouth or burnished, expressed as the ratio of percentage elongation to yield strength.
Poisson’s ratio, v: ratio of lateral to axial strain under tensile loading. It denotes reduction in cross-section during elongation.
Brittle materials have low υ values, i.e. little change in cross-section with elongation, while ductile materials show a greater reduction in cross-section, known as specimen necking.
Elastic modulus, E is the ratio of stress to strain. It is also known as modulus of elasticity or Young’s modulus and denotes material stiffness. It is determined as the slope of the elastic (linear) portion of the stress–strain curve.
Stress–strain curves are generated by applying a progressively increasing tensile force while measuring applied stress and material strain until fracture occurs. The shape of the curve indicates the properties of the material (Figures 1.3 and 1.4). Non-ferrous metals (e.g. gold and copper) show a continuous curve to failure while ferrous materials exhibit a ‘kink’ in the curve, known as the yield point.
The intersection of a line parallel to the abscissa (strain) axis from the failure point to the ordinate (stress) axis is specimen strength while the vertical line from the failure point to the strain axis is the ductility.
High strength, brittle materials show steep stress–strain curves with little strain at failure, e.g. ceramics.
Strong ductile materials (e.g. metals) show moderate slopes in the stress–strain curve but good extension until failure.
Soft ductile materials (e.g. elastomers) show long, shallow linear stress–strain behavior followed by a sharp rise in the curve when, with increasing applied force, the elastomer no longer extends linearly (or elastically) and failure occurs.
Resilience: resistance to permanent deformation (i.e. energy required for deformation to the proportional limit). It is given by the area under the elastic portion of the stress–strain curve (Figure 1.5).
Toughness: resistance to fracture (i.e. energy required to cause fracture). It is given by the total area (i.e. both the elastic and plastic regions) under the stress–strain curve (Figure 1.5).
Figure 2.1 Optimal loading (stress and strain) region for a resilient material.

Figure 2.2 Diametral disc test for determining the tensile strength of brittle materials.

Figure 2.3a Transverse testing of a specimen.

Figure 2.3b Loads and resultant stresses in a specimen under transverse testing.

Fracture toughness is the ability to deform plastically without fracture and it is proportional to energy consumed in plastic deformation. Cracks or flaws, arising naturally or developing over time, cause weakening such that fracture may occur at stresses below the yield stress, the flaw acting as a stress riser.
Flaws cause problems because brittle materials under loading cannot deform plastically and redistribute stresses. As the flaw or crack size increases, the stress to specimen failure decreases. This behavior is expressed by the stress intensity factor K which is determined by the stress and the crack length. Fracture occurs when the stress intensity reaches a critical value, Kc given by Y·σ· √πa, where Y is a function of crack size and geometry, and a is crack length.
This critical value is known as the fracture toughness of the material.
Elastic materials deform when loaded but when the load is released, the specimen will resume its original dimensions although the recovery rate varies with the material. Deformation (strain) is directly proportional to the applied load (stress) in accordance with Hooke’s law up to the proportional limit.
Plastic materials deform when loaded but the deformation is not proportional to the applied load. Upon release of the applied force, the specimen does not completely recover its original dimensions and is said to be plastically deformed.
Subjecting an elastic material to a load above its elastic limit will induce a degree of plastic (permanent) deformation. Ideally, applied loads should never exceed the elastic limit (Figure 2.1).
Tensile properties (Chapter 1) are measured on flat specimens with a ‘necked’ region or dumbbell-shaped specimens. Brittle materials (e.g. amalgam and ceramics) cannot be tested in tension, and tensile properties are determined by the diametral tensile test. In this test, a compressive load (P) is applied to a vertical disc of material and induces a tensile force along the specimen diameter (Figure 2.2). The diametral tensile strength (DTS) is given by:
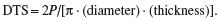
Compressive strength is determined by applying a compressive load to a cylindrical or square cross-section specimen and is expressed as the load to failure divided by cross-sectional area.
Shear strength is determined by applying a tensile stress to a lapped specimen, by a modified cantilever test or a pin-disc system and is important when shear loading occurs (e.g. with veneers).
Transverse strength: a specimen of length L is supported near the ends with a load (P) applied in the middle (Figure 2.3). Failure initiates at the lower edge where the applied force induces tensile stresses while compressive forces occur in the upper region. Strength is given by stress at failure:
strength, and deformation,
and deformation,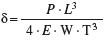
where E is the modulus, W is the width and T is the thickness.
This characteristic is important for denture bases.
Indentation hardness is resistance to penetration and is a measure of scratch resistance. Hardness is measured by several techniques, including the Barcol, Bierbaum, Brinell, Knoop, Rockwell, Shore and Vickers tests. Of these, the most important in dentistry are the Knoop and Shore tests.
The Knoop hardness test uses a non-symmetrical diamond point (7 : 1 ratio of length diagonal to width diagonal), and the Knoop hardness number KHN = L/12 · CK, where L is the applied load, l is the length of the long diagonal and CK is a constant that relates l to the indentation area. The test requires a flat, highly polished specimen but no load is specified so it can be used on a microscopic scale for both ductile and brittle materials.
Shore hardness measures penetration of a blunt indenter into a soft or elastic material and is useful for soft materials such as elastomeric materials.
Hardness values can provide an indication of the resistance of materials to wear and abrasion.
Abrasion and wear are important for polymeric restorations, ceramic restorations opposing natural teeth and dentifrices. Surface hardness is not always a reliable guide to wear resistance, particularly for hard, brittle materials or for elastomers. Various abrasion/wear test systems are used, the simplest being reciprocating arm abraders with nylon brushes or rubber cups mounted on the ends of counterbalanced arms that are driven over the test piece. Weights placed on the arm vary the applied load while water, artificial saliva or dentifrice slurries can be applied to the test piece surface. More complex test arrangements have specimens mounted on or subjected to rotating or oscillating heads, again with abrasives applied to test specimen surface. Wear/abrasion damage is assessed by profilometry (change in the surface profile), weight loss or both.
No abrasion system completely mimics behavior in the oral cavity and data quantification and reproducibility can both present problems. Nevertheless, abrasion/wear testing can provide useful predictive data with regard to material performance.
Figure 3.1 Effect of temperature rise on a restoration and tooth with different coefficients of thermal expansion.

Table 3.1 Thermal properties of various dental materials
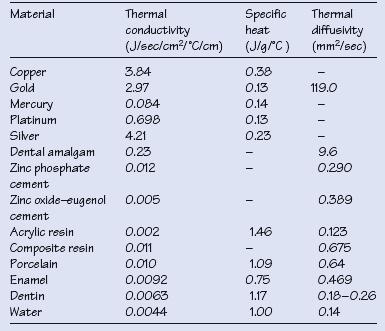
Table 3.2 Coefficients of thermal expansion.
| material | Coefficient of thermal expansion (*10−6/°C) |
| Tooth (crown portion) | 11.4 |
| Amalgam | 22.1–28.0 |
| Gold | 14.4 |
| Composite resin | 17–50 |
| Acrylic resin | 76.0 |
| Porcelain | 12.0 |
| Glass ionomer | 10.2–11.4 |
| Inlay wax | 350–450 |
| Silicone impression material | 210 |
| Polysulfide impression material | 140 |
Table 3.3 Electrical constants for dental materials and teeth.
| Material | Resistivity(ohm · cm) | Dielectric constant |
| Tooth enamel | 2.6–6.9 × 106 | – |
| Dentin | 1.1–5.2 × 104 | 8.6 |
| Glass ionomer | 0.8–2.5 × 104 | 2–7 × 105 |
| Zinc oxide–eugenol cement | 109–1010 | 10 |
| Zinc polyacrylate cement | 0.4–4 × 105 | 4 × 103–2 * 105 |
| Zinc phosphate cement | 2 × 105 | – |
Table 3.4 Wavelengths of visible light.
| Color | Approximate wavelength interval (nm) |
| Red | 630–700 |
| Orange | 590 –630 |
| Yellow | 560–590 |
| Green | 490 –560 |
| Blue | 450– 490 |
| Indigo | 420 – 440 |
| Violet | 400–450 |
Physical properties of relevance to dental biomaterials include thermal, electrical and optical properties; typical values are given in Table 3.1.
Thermal conductivity, K: rate of heat conduction through a unit cube of material when the temperature difference across the cube is 1°C.
Metals have higher K values than teeth and polymers, and cause greater temperature changes within the dental pulp with hot or cold liquids and foodstuffs.
Specific heat, Cp: quantity of heat needed to raise the temperature of 1 g of substance by 1°C.
Specific heat is important during melting and casting since it determines the heat input required to reach the melting point of a metal. Cp is low for gold and higher for non-precious and base metal alloys, indicating that more heat is required to melt these metals.
Thermal diffusivity, Δ is defined as K/Cp × ρ