

Contents
Preface To The First Edition
Preface To The Second Edition
Preface To The Third Edition
CHAPTER 1 PRESURGICAL CONSIDERATIONS
Preoperative Evaluation of the Patient
Surgical Judgment
Principles of Asepsis and Antisepsis
Surgical Classifications
Role of Antibiotics
Preoperative Planning
Preparation of the Surgical Site
Postoperative Infection
CHAPTER 2 ANESTHESIA AND FLUID THERAPY
Anesthesia
Fluid Therapy
CHAPTER 3 SURGICAL INSTRUMENTS
Use of Surgical Instruments
Preparation of Instruments
General Surgical Instruments
Instruments Used Specifi cally in Large Animal Surgery
CHAPTER 4 SUTURE MATERIALS AND NEEDLES
Suture Materials
Needles
CHAPTER 5 KNOTS AND LIGATURES
Principles of Knot Tying
Ligatures
CHAPTER 6 SUTURE PATTERNS
Basic Suture Patterns
Suture Patterns Used for Closure of Hollow Organs
Stent Bandages (Tie-Over Dressings)
Suture Patterns for Severed Tendons
CHAPTER 7 PRINCIPLES OF WOUND MANAGEMENT AND THE USE OF DRAINS
Wound Management
Methods of Closure and Healing
Use of Drains
CHAPTER 8 RECONSTRUCTIVE SURGERY OF WOUNDS
Elliptical Excision Undermining for Repair of an Elongated Defect
Wound Closure Using Tension-Relieving Incisions
Sliding H-Flap
Z-Plasty
Removal of Excessive Scar Tissue
Skin Grafting
CHAPTER 9 EQUINE ORTHOPEDIC SURGERY
Medial Patellar Desmotomy
Cunean Tenectomy
Lateral Digital Extensor Tenotomy
Inferior (Distal) Check Ligament Desmotomy
Superior Check Ligament Desmotomy (After Bramlage)
Superfi cial Digital Flexor Tenotomy
Deep Digital Flexor Tenotomy
Sectioning of the Palmar (or Plantar) Annular Ligament of the Fetlock
Palmar Digital Neurectomy
Amputation of the Splint (II and IV Metacarpal and Metatarsal) Bones
Arthrotomy of the Midcarpal Joint
Arthrotomy of the Fetlock Joint and Removal of an Apical Sesamoid Chip Fracture
CHAPTER 10 EQUINE UROGENITAL SURGERY
Castration
Cryptorchidectomy by the Inguinal and Parainguinal Approach
Laparoscopic Cryptorchidectomy
Caslick’s Operation for Pneumovagina in the Mare
Urethroplasty by Caudal Relocation of the Transverse Fold
Cesarean Section in the Mare
Circumcision of the Penis (Reefi ng)
Amputation of the Penis
Aanes’ Method of Repair of Third-Degree Perineal Laceration
CHAPTER 11 SURGERY OF THE EQUINE UPPER RESPIRATORY TRACT
Tracheostomy
Laryngotomy, Laryngeal Ventriculectomy, and Ventriculocordectomy
Partial Resection of the Soft Palate
Surgical Entry and Drainage of the Guttural Pouches
CHAPTER 12 EQUINE DENTAL AND GASTROINTESTINAL SURGERY
Repulsion of Cheek Teeth
Ventral Midline Laparotomy and Abdominal Exploration
Standing Flank Laparotomy
Umbilical Herniorrhaphy in the Foal
CHAPTER 13 BOVINE GASTROINTESTINAL SURGERY
Principles of Laparotomy
Flank Laparotomy and Abdominal Exploration
Rumenotomy
Rumenostomy (Rumenal Fistulation)
Surgical Corrections of Abomasal Displacements and Torsion
CHAPTER 14 BOVINE UROGENITAL SURGERY
Calf Castration
Urethrostomy
Hematoma Evacuation of the Bovine Penis
Preputial Amputation (Circumcision) in the Bull
Surgical Techniques for Teaser Bull Preparation
Inguinal Herniorrhaphy in the Mature Bull
Cesarean Section in the Cow
Retention Suturing of the Bovine Vulva (Buhner’s Method)
Cervicopexy for Vaginal Prolapse (After Winkler)
CHAPTER 15 MISCELLANEOUS BOVINE SURGICAL TECHNIQUES
Digit Amputation
Eye Enucleation
Cosmetic Dehorning
Rib Resection and Pericardiotomy
Repair of Teat Lacerations
CHAPTER 16 SURGICAL TECHNIQUES IN SWINE
Castration of the Piglet
Inguinal Herniorrhaphy in the Piglet
Cesarean Section in the Sow
CHAPTER 17 MISCELLANEOUS SURGICAL TECHNIQUES
Dehorning the Mature Goat
Tooth Removal in the Llama
Index

Dean A. Hendrickson, DVM, MS, DACVS, is a professor of large animal surgery at Colorado State
University College of Veterinary Medicine, Fort Collins, Colorado.
© 2007 Blackwell Publishing
All rights reserved
Blackwell Publishing Professional
2121 State Avenue, Ames, Iowa 50014, USA
Orders: 1-800-862-6657
Office: 1-515-292-0140
Fax: 1-515-292-3348
Web site: www.blackwellprofessional.com
Blackwell Publishing Ltd
9600 Garsington Road, Oxford OX4 2DQ, UK
Tel.: +44 (0)1865 776868
Blackwell Publishing Asia
550 Swanston Street, Carlton, Victoria 3053, Australia
Tel.: +61 (0)3 8359 1011
Authorization to photocopy items for internal or personal use, or the internal or personal use of specific clients, is granted by Blackwell Publishing, provided that the base fee is paid directly to the Copyright Clearance Center, 222 Rosewood Drive, Danvers, MA 01923. For those organizations that have been granted a photocopy license by CCC, a separate system of payments has been arranged. The fee code for users of the Transactional Reporting Service is ISBN-13: 978-0-7817-8255-5/2007.
First and Second editions, © Lea & Febiger
Third edition, © Blackwell Publishing
Library of Congress Cataloging-in-Publication Data
Hendrickson, Dean A.
Techniques in large animal surgery. – 3rd ed. / by Dean A. Hendrickson.
p. ; cm.
Rev. ed. of: Techniques in large animal surgery / A. Simon Turner, C. Wayne McIlwraith. 2nd ed. 1989. Includes bibliographical references and index.
ISBN 978-0-7817-8255-5 (alk. paper)
1. Veterinary surgery. 2. Horses–Surgery. 3. Cattle–Surgery. I. Turner, A. Simon (Anthony Simon) Techniques in large animal surgery. II. Title.
[DNLM: 1. Surgery, Veterinary–methods. 2. Goats–surgery. 3. Horses–surgery. 4. Surgical
Procedures, Operative–veterinary. 5. Swine–surgery. SF 911 H498t 2007]
SF911.T87 2007
636.089'7–dc22
2007019840
PREFACE TO THE FIRST EDITION
The purpose of this book is to present some fundamental techniques in large animal surgery to both veterinary students and large animal practitioners. It is designed to be brief, discussing only the major steps in a particular operation, and each discussion is accompanied by appropriate illustrations. Most of the techniques presented in this book can be performed without the advantages of a fully equipped large animal hospital or teaching institution.
The book assumes a basic understanding of anatomy and physiology. Those who wish to know more about a particular technique are encouraged to consult the bibliography.
We and our colleagues at the Colorado State University Veterinary Teaching Hospital consider the procedures discussed in this book to be time honored. Some practitioners may perform certain techniques in slightly different ways. We would be happy to receive input about modifications of these techniques for future editions of this book.
All of the drawings in the book are original and based on rough sketches and photographs taken at various points during actual surgery. Occasionally, dissections were performed on cadavers.
The surgical procedures described in this text represent not only our thoughts, but suggestions from many of our colleagues as well. Their help was an important contribution to the production of this book. We are indebted to Dr. Wilbur Aanes, Professor of Surgery, Colorado State University, who unselfishly shared 30 years of his personal experience in large animal surgery with us. We are proud to be able to present in Chapter 10 of this book “Aanes’ Method of Repair of Third-Degree Perineal Laceration” in the mare, a technique that he pioneered over 15 years ago. We also wish to give credit to the following faculty members at Colorado State University Veterinary Teaching Hospital who willingly gave us advice on the diagrams and manuscript of various techniques discussed in this book: Dr. Leslie Ball, Dr. Bill Bennett, Dr. Bruce Heath, Dr. Tony Knight, Dr. LaRue Johnson, Dr. Gary Rupp, Dr. Ted Stashak, Dr. Gayle Trotter, Dr. James Voss, and Dr. Mollie Wright. We also wish to
express appreciation to Dr. John Baker, Purdue University, and Dr. Charles Wallace, University of Georgia, for their comments on some questions we had. Dr. McIlwraith is also grateful to Dr. John Fessler, Professor of Surgery, Purdue University, for his inspiration and training.
We are particularly grateful to Dr. Robert Kainer, Professor of Anatomy, Colorado State University, for checking the manuscript and the illustrations and advising us on nomenclature. His input impressed upon us the importance of the relationship between the dissection room and the surgery room.
The terrific amount of time and effort involved with the illustrations will be clear to the reader who cares only to leaf through the book. For these illustrations, we are indebted to Mr. Tom McCracken, Director, Office of Biomedical Media, Colorado State University. We are thankful for his expertise, as well as his cooperation and understanding. The diagrams for “Aanes’ Method of Repair of Third-Degree Perineal Laceration” were done by Mr. John Daughtery, Medical Illustrator, Colorado State University. We must also thank Kathleen Jee, who assisted with various aspects of the artwork. We would also like to thank Messrs. Al Kilminster and Charles Kerlee for taking photographs during the various surgical procedures that were used to assist with the artwork of this text.
The manuscript was typed by Mrs. Helen Mawhiney, Ms. Teresa Repphun, and Mrs. Jan Schmidt. We thank them for their patience and understanding during the many changes we made during the generation of the final manuscript.
We are grateful to the following instrument companies for allowing us to use some of the diagrams from their sales catalogs for inclusion in Chapter 3, “Surgical Instruments”: Schroer Manufacturing Co., Kansas City, MO; Intermountain Veterinary Supply, Denver, CO; Miltex Instrument Co., Lake Success, NY; J. Skyler Manufacturing Co., Inc., Long Island, NY.
The idea for this book was conceived in 1978 when one of us (AST) was approached by Mr. George Mundroff, Executive Editor, Lea & Febiger. We would like to thank him for his encouragement and guidance. We are also grateful to Mr. Kit Spahr, Jr., Veterinary Editor; Diane Ramanauskas, Copy Editor; Tom Colaiezzi, Production Manager; and Samuel A. Rondinelli, Assistant Production Manager, Lea & Febiger, for their assistance, as well as to others at the Publisher who assisted in the production of this book.
A. Simon Turner
C. Wayne McIlwraith
Fort Collins, Colorado
PREFACE TO THE SECOND EDITION
The second edition of Techniques in Large Animal Surgery is in response to the acceptance of the first edition and the continued need for such a book for both veterinary students and large animal practitioners. In many instances, the techniques are time honored and require no change from 5 years ago. In other instances, however, refinements in technique as well as improved perception of indications, limitations, and complications have made changes appropriate.
A significant change is the addition of Dr. R. Bruce Hull, Professor of Veterinary Clinical Sciences, The Ohio State University, as a contributor. He has carefully analyzed the entire bovine section, and his suggested changes and additions have been incorporated into the text. In addition, two procedures, “teaser bull preparations by penile fixation” and “treatment of vaginal prolapse by fixation to the prepubic tendon,” have been added. We are most grateful in having Dr. Hull’s help and expertise. Among the introductory chapters, the section on anesthesia required the most updating, and we are grateful to our colleague Dr. David Hodgson at Colorado State University for his review and advice. Two new procedures, “superior check ligament desmotomy” and “deep digital flexor tenotomy,” were considered appropriate additions to this edition. We are grateful to Dr. Larry Bramlage, Ohio State University, for his comments and help with the first of these procedures. Many of the other changes in this edition are in response to the book reviews and comments on the first edition returned to Lea & Febiger. To these people, we appreciate your feedback.
A chapter on llama tooth removal was added because of the increased popularity of this species, especially in our own part of the country. Although we only discuss this one technique, it should not be inferred that other operations are unheard of in llamas. We have corrected angular limb deformities, repaired fractures, and performed gastrointestinal surgery, among other procedures, but tooth removal is the most common. Descriptions of these other procedures in llamas are beyond the scope of this book at this stage.
The need for more sophisticated equine techniques prompted us to produce the textbook Equine Surgery: Advanced Techniques in 1987. It is envisioned that the book will be used as a companion to this second edition, to provide a full spectrum of equine procedures, with the well-accepted format of concise text and clear illustrations.
Again, we are thankful to Mr. Tom McCracken, Assistant Professor, Department of Anatomy and Neurobiology, Colorado State University, for his talent in capturing the techniques described in his line drawings. We are also indebted to Helen Acvedo for typing our additions and to Holly Lukens for copyediting. Finally, our thanks again to the excellent staff at Lea & Febiger for the production of this edition.
A. Simon Turner
C. Wayne McIlwraith
Fort Collins, Colorado
PREFACE TO THE THIRD EDITION
The first two editions of Techniques in Large Animal Surgery have been well accepted, much to the credit of Drs. Turner and McIlwraith. They have been excellent texts for the veterinary student and the large animal practitioner. I was fortunate to be able to take on the task when it came time to update the information for a third edition. I am deeply appreciative of the opportunity to take such an excellent text and update it with new information and techniques.
The third edition of Techniques in Large Animal Surgery has been updated in response to the continued need for such a book for both veterinary students and large animal practitioners. There are some techniques that are time tested and continue to be included. There are other techniques that have been refined or replaced, and these are included in the new text.
New information has been included in essentially every chapter. We have made extensive use of tables to simplify the information. The anesthesia section includes new and updated information on sedation and anesthetic agents. The instrument section has been evaluated, adding new
instruments where applicable and removing outdated or unavailable instruments. The section on suture materials has been updated to include new materials. There are new illustrations in the suture pattern section to better aid the practitioner with surgical techniques. The sections on wound management and reconstructive surgery have been increased to provide up-to-date information on wound care. Tables of required instrumentation have been added to all sections of the remaining surgical chapters to aid in surgical planning and preparation.
I am very grateful for our new illustrator Anne Rains; she has done an excellent job and has made my life very easy. I am indebted to Joanna Virgin who has done the lion’s share of the research to make sure this text was as up-to-date and accurate as possible. I could not have done this work without her. Thanks to the folks at Blackwell for their help and assistance in the production of this edition.
Dean A. Hendrickson
Fort Collins, Colorado
Objectives
Before a surgical procedure, a physical examination is generally indicated. This applies to both emergency and elective surgery. The following are laboratory tests that are generally indicated for horses based upon animal age and systemic status at our clinic:
Exactly where to draw the line on laboratory tests is largely a matter of judgment on the part of the surgeon. Obviously, if the surgery consists of castration of several litters of piglets, then for purely economic reasons laboratory tests prior to surgery will not be performed. In many cases, however, additional tests will be necessary. The following are examples of other optional tests and their indications:
If any laboratory parameters are abnormal, the underlying causes should be investigated and efforts made to correct them. In “elective” surgery this is possible, but it may not be possible in an emergency. The owner should be made aware of any problems prior to subjecting the animal to surgery. Risks are always present in normal elective surgery, and these should be explained to the owner.
Fluid replacement should be performed if necessary. In the elective case, the surgical procedure should be postponed if the animal’s physical condition or laboratory parameters are abnormal. In some animals, internal and external parasitism may have to be rectified to achieve this goal.
Medical records should be kept at all times. Obviously this can be difficult in such cases as castration of several litters of piglets, but record keeping should become an essential part of the procedure for horses and cattle in a hospital and herd records should be kept in all other situations. Finally, if the animal is insured, the insurance company must be notified of any surgical procedure; otherwise, the policy may be void.
Surgical judgment cannot be learned overnight by reading a surgery textbook, nor is it necessarily attained by years of experience. The surgeon who continually makes the same mistake will probably never possess good surgical judgment. Not only should the surgeon learn from his own mistakes; he also should learn from the mistakes of others, including those documented in the surgical literature. As part of surgical judgment, the surgeon must ask the following questions:
Is the surgery necessary?
What would happen if the surgery were not performed?
Is the procedure within the capabilities of the surgeon, the facilities, and the technical help?
What is the economical and/or sentimental value of the animal; does it outweigh or reinforce the cost of the surgery?
If the surgeon finds that the procedure is too advanced for his or her capabilities and/or facilities, the surgery should be referred. Some veterinarians have a fear that this will mean loss of the client’s business in the future, but this is rarely the case. If the surgeon explains why the case should be referred elsewhere, most clients will be grateful for such frankness and honesty. It is inexcusable to operate on a patient and then have complications arise due to inadequate training and facilities, when the surgery could easily have been referred to a well-equipped, wellstaffed hospital with specially qualified personnel. Clearly, this rule has exceptions—mainly the emergency patient, which may fare better by undergoing immediate surgery than being subjected to a long trailer ride to another facility.
Many of the procedures described in this book can be done “on the farm.” Some, such as arthrotomy for removal of chip fractures of the carpal and sesamoid bones in horses, should be done in a dust-free operating theater. If clients want these latter procedures to be done “in the field,” they should understand the disastrous consequences of postsurgical infection. The surgeon must be the final judge of whether his facilities or experience are suitable.
There are three determinants of an infection in a surgical site: host defense, physiologic derangement, and bacterial contamination risk at surgery.2 Control methods include aseptic surgical practices as well as identification of the high-risk patient, correction of systemic imbalances prior to surgery, and the proper use of prophylactic antibiotics.3
We are sometimes reminded by fellow veterinarians in the field that we must teach undergraduates how to do surgery in the real world. By this they mean that we must ignore aseptic draping and gloving and lower the standard to a “practical” level. This is fallacious in our opinion. Although we recognize that the ideal may be unattainable in private practice, one should always strive for the highest possible standard; otherwise, the final standard of practice may be so low that the well-being of the patient is at risk, not to mention the reputation of the veterinarian as a surgeon. For this reason, we believe that it behooves us as instructors of the undergraduate to teach the best possible methods with regard to asepsis as well as technique.
Table 1-1. Surgical classifications.
| Classification | Description | Examples |
| Clean | Gastrointestinal, urinary, or respiratory tract is not entered. | Arthrotomy for removal of a chip fracture of a carpal bone of a horse |
| Clean-contaminated | Gastrointestinal, respiratory, or urinary tract is entered. There is no spillage of contaminated contents. | Abomasopexy for displaced abomasums in the dairy cow |
| Contaminated-dirty | Gross spillage of contaminated body contents or acute inflammation occurs. | Wounds Abscesses Devitalized bowel |
The extent to which the practice of asepsis or even antisepsis is carried out depends on the classification of the operation, as shown in Table 1.1. This classification may also help the veterinarian decide whether antibiotics are indicated or whether postoperative infection can be anticipated.
Once the surgeon has categorized the surgical procedure, appropriate precautions to avoid postoperative infection can be determined. In all cases, however, the surgical site is prepared properly, including clipping and aseptic scrubbing.
Whatever category of surgery is performed, clean clothing should be worn. The wearing of surgical gloves is good policy even if to protect the operator from infectious organisms that may be present at the surgical site. Surgical gowns, gloves, and caps are recommended for clean surgical procedures, although such attire has obvious practical limitations for the large animal surgeon operating in the field. The purpose of this book is to present guidelines rather than to lay down hard-and-fast rules. For example, the decision between wearing caps, gowns, and gloves and wearing just gloves can be made only by the surgeon. Good surgical judgment is required. In general, it is better to be more careful than what may appear necessary in order to be better prepared when problems arise.
Antibiotics should never be used to cover flaws in surgical technique. The young surgeon is often tempted, sometimes under pressure from the client, to use antibiotics prophylactically. However, the disadvantages of antimicrobial therapy often outweigh its benefits. Extended periods of antimicrobial therapy can select for resistant organisms and adversely affect the gastrointestinal tract by eliminating many of the normal enteric organisms and allowing outgrowths of pathogenic bacteria, such as Clostridia spp., which can result in colitis and diarrhea.4 When selecting an antibiotic regimen, the surgeon should consider the following aspects:
Does the diagnosis warrant antibiotics?
Which organisms are most likely to be involved and what is their in vitro antimicrobial susceptibility?
What is the location or likely location of the infection?
How accessible is the location of the infection to the drug?
What possible adverse reactions and toxicities to the drug could occur?
What dosage and duration of treatment are necessary to obtain sufficient concentrations of the drug?
Again, some judgment is required, but suffice to say, antibiotics should never be a substitute for “surgical conscience.” Surgical conscience consists of the following: dissection along tissue planes, gentleness in handling tissues, adequate hemostasis, selection of the best surgical approach, correct choice of suture material (both size and type), closure of dead space, and short operating time.
If the surgeon decides that antibiotics are indicated, special attention should be given to selecting the type of antimicrobial drug, dosage, and duration of use. Ample scientific literature indicates that for maximum benefit, antimicrobials should be administered prophylactically prior to surgery and, at the latest, during surgery. Beyond 4 hours postsurgically, the administration of prophylactic antibiotics has little to no effect on the incidence of postoperative infection.1 The duration of treatment should not exceed 24 hours because most research indicates that antimicrobial use after this period of time does not confer further benefits. If longer duration of antimicrobial coverage is necessary, the full duration of the specific antimicrobial drug selected should be given. This varies depending on the drug; however, in most cases the duration is at least 3 to 5 days. If the surgeon is operating on a food animal, there are regulations for withdrawal times from different antimicrobial drugs prior to slaughter that must be taken into account.
If topical antibiotics are used during surgery, they should be nonirritating to the tissues; otherwise, tissue necrosis from cellular damage will outweigh any advantageous effects of the antibiotics. It is also beneficial when using topical antibiotics to use antibiotics that are not generally used systemically.
All equine surgical patients should have tetanus prophylaxis. If the immunization program is doubtful, the horse can receive 1500 to 3000 units tetanus antitoxin. Horses on a permanent immunization program that have not had tetanus toxoid within the previous 6 months should receive a booster injection.
Tetanus prophylaxis is generally not provided for food animals, but an immunization program may be considered, especially if a specific predisposition is thought to exist.
The surgeon should be thoroughly familiar with the regional anatomy. In this book we illustrate what we consider to be the important structures in each technique. If more detail is required, a suitable anatomy text should be consulted. Not only should the procedure be planned prior to the surgery, but the surgeon also should visit the dissection room and review local anatomy on cadavers prior to attempting surgery on a client’s animal. We are fortunate in veterinary surgery to have greater access to cadavers than our counterparts in human surgery.
For the large animal surgeon, preparation of the surgical site can present major problems, especially in the winter and spring when farms can be muddy. Preparation for surgery may have to begin with removal of dirt and manure. Some animals that have been recumbent in mud and filth for various reasons may have to be hosed off. Hair should then be removed, not just from the surgical site, but from an adequate area surrounding the surgical site.
The clipping should be done in a neat square or rectangular shape with straight edges. Surprisingly, this, along with the neatness of the final suture pattern in the skin, is how the client judges the skill of the surgeon. Clipping may be done initially with a no. 10 clipper blade, and then the finer no. 40 blade may be used. The incision site can be shaved with a straight razor in horses and cattle, but debate exists regarding the benefit or problems associated with this procedure. In sheep and goats, in which the skin is supple and pliable, it is difficult to shave the edges.
Preparation of the surgical site, such as the ventral midline of a horse about to undergo an exploratory laparotomy, may have to be performed when the animal is anesthetized. If surgery is to be done with the animal standing, an initial surgical scrub, followed by the appropriate local anesthetic technique and a final scrub, is standard procedure.
For cattle or pigs, the skin of the surgical site can be prepared for surgery with the aid of a stiff brush. For the horse, gauze sponges are recommended. Sheep may require defatting of the skin with ether prior to the actual skin scrub. The antiseptic scrub solution used is generally a matter of personal preference. Either povidone-iodine scrub (Betadine Scrub) alternated with a 70% alcohol rinse, or Chlorhexidine alternated with water, can be used. Finally, the skin can be sprayed with povidoneiodine solution (Betadine Solution) and allowed to dry.
Scrubbing of the proposed surgical site is done immediately prior to the operation. Scrubbing should commence at the proposed site of the incision and progress toward the periphery; one must be sure not to come back onto a previously scrubbed area. Some equine surgeons clip and shave the surgical site the night before the surgery, perform an aseptic preparation as previously described, and wrap the limb in a sterile bandage until the next day. A shaving nick made the day before surgery may be a pustule on the day of surgery, however, and this is generally not recommended for anything proximal to the pastern region.
When aseptic surgery is to be performed, an efficient draping system is mandatory. Generally, time taken to drape the animal properly is well spent. The draping of cattle in the standing position can be difficult, especially if the animal decides to move or becomes restless. It can be difficult to secure drapes with towel clamps in the conscious animal because only the operative site is anesthetized. If draping is not done, the surgeon must minimize contact with parts of the animal that have not been scrubbed. The tail must be tied to prevent it from flicking into the surgical field.
Several operations described in this book require the strictest of aseptic technique, and sterile, antimicrobial, adhesive, incise drapes are indicated. Characteristics of sterile plastic adhesive drapes include their ability to adhere, their antimicrobial activity, and their clarity when applied to the skin. Probably the most desirable feature is the one first mentioned. With excessive traction or manipulation, some brands of drapes quickly separate from the skin surfaces, and this separation instantly defeats their purpose.
Rubberized drapes are helpful when large amounts of fluids (such as peritoneal and amniotic fluid) are encountered during the procedure. Rubberized drapes are also useful to isolate the bowel or any other organ that is potentially contaminated, to prevent contamination of drapes. Newer fluid-impermeable paper drapes that are disposable make the surgeon’s job even easier.
Prevention of postoperative infection should be the goal of the surgeon, but infection may occur despite all measures taken to prevent it. If infection occurs, the surgeon must decide whether antibiotic treatment is indicated, or whether the animal is strong enough to fight it using its own defense mechanisms. Some surgical wounds require drainage at their most ventral part, whereas others require more aggressive treatment. If, in the judgment of the surgeon, the infection appears to be serious, a Gram stain, culture, and sensitivity testing of the offending microorganism(s) will be indicated. A Gram stain may give the surgeon a better idea of what type of organism is involved and may in turn narrow the selection of antibiotics. Sometimes in vitro sensitivities have to be ignored because the antibiotic of choice would be prohibitively expensive. This is especially true for adult cattle and horses. A broad-spectrum antibiotic should be given, if possible, as soon as practical.
References
1. Burke, J.F.: Preventing bacterial infection by coordinating antibiotic and host activity. In Symposium on prophylactic use of antibiotics. South Med. J., 70:24, 1977.
2. Cristou, N.V., Nohr, C.W., and Meakins, J.L.: Assessing operative site infection in surgical patients. Arch. Surg., 122:165, 1987.
3. Nelson, C.L.: Prevention of sepsis. Clin. Orthop., 222:66, 1987.
4. Papich, M.G.: Antimicrobial therapy for gastrointestinal disease. Vet. Clin. Eq., 19:645–663, 2003.
The purpose of this section is not to present an in-depth discussion of anesthesia. Details on the principles of anesthesia, recognition of stages of anesthesia, monitoring, and the pharmacology and physiology associated with anesthesia are well documented in other texts.34,58,77 In this section, anesthetic techniques used routinely by us are presented. Many alternatives are available and personal preferences differ, but we consider these to be suitable for the individual surgical techniques presented in this textbook.
Local or infiltration anesthesia is the injection of a surgical site directly with analgesic agent. Regional anesthesia is desensitization by blocking the major nerve(s) to a given region. Both techniques permit the desensitization of the surgical site. Because they are purely analgesic techniques, the term analgesia is preferred to the term anesthesia. The two analgesic agents most commonly used are 2% lidocaine hydrochloride (Lidocaine Hydrochloride Injection 2%) and 2% mepivacaine hydrochloride (Carbocaine). Although lidocaine has essentially replaced procaine hydrochloride as the standard local analgesic agent, mepivicaine is also widely used because of its rapid onset, longer duration, and less associated tissue reaction.7
In the ox in particular, surgical procedures are commonly performed under local or regional analgesia. In many instances, surgery is performed on the standing animal, and no sedation is used. In other instances, a combination of sedation and casting is used in conjunction with a local analgesic regimen. Local and regional analgesic techniques that are used routinely in individual species follow.
The principles of infiltration analgesia are simple and are similar for all species. The limits of the region to be infiltrated may be well defined by making a subcutaneous wheal. A small amount of analgesic agent is injected at an initial site with a small needle and then, if a long region of analgesia is required, a longer needle is inserted through the initial region of desensitization. Needles should always be reinserted through a region that has already been infiltrated. The skin and subcutis should be infiltrated first and then the deeper layers, such as muscle and peritoneum. Avoid the injection of significant amounts of analgesic solution into the peritoneal cavity; rapid absorption can take place, with the possibility of resultant toxicity. Infiltrating injections should be made in straight lines, and “fanning” should be avoided as much as possible because of the tissue trauma it causes.
Infiltration analgesia is commonly used for suturing wounds and for removing cutaneous lesions in all large animal species. It may also be used in the form of a line block for laparotomy, in which case the analgesic agent is infiltrated along the line of incision. Although convenient, the infiltration of analgesic agent into the incision line causes edema in the tissues and may affect wound healing. In this respect, regional analgesic techniques are generally considered preferable.
Inverted L block is the simplest technique of regional analgesia for laparotomy and laparoscopy approaches in large animal species. It may be used for either flank or paramedian laparotomies, laparoscopic procedures such as cryptorchidectomies and ovariectomies, and urogenital surgery. The principles of the technique are illustrated in the ox in Figure 2-1. It is a nonspecific technique in which local analgesic agent is deposited in the form of an inverted L to create a wall of analgesia enclosing the surgical field. All nerves entering the operative field are blocked. The procedure is facilitated by the use of an 8- to 10-cm, 16- to 18-gauge needle. Up to 100 ml of local analgesic agent may be used in an adult-sized horse or cow; however, the author recommends using no more than 60 ml. The vertical line of the L passes caudal to the last rib, and the horizontal line is just ventral to the transverse processes of the lumbar vertebrae. Ten to fifteen minutes should be allowed for the analgesic agent to take effect.
Special consideration should be given to regional and local analgesia in sheep and goats. Systemic toxicity is a potential complication with these species, and dosage limits should be considered. Experiments in sheep have shown that convulsions occur in adult sheep at a dose of lidocaine hydrochloride of 5.8 ± 1.8 mg/kg intravenously.59 Subconvulsive doses of lidocaine hydrochloride often produce drowsiness, however. Above convulsive doses, hypotension occurs at 31.2 ± 2.6 mg/kg, respiratory arrest at 32.4 ± 2.8 mg/kg, and circulatory collapse at 36.7 ± 3.3 mg/kg. An initial dose of 6 mg/kg is within a reasonable margin to avoid serious complications. If convulsions do occur, they can be controlled with an intravenous dose of 0.5 mg/kg of diazepam (Valium). Diluted solutions of lidocaine in local blocks of sheep and goats are advantageous in these species.27,34
The paravertebral block is not commonly used in equine species, but is frequently performed in cattle, sheep, and goats.10,27 In ruminants, the thirteenth thoracic nerve (T13), the first and second lumbar nerves (L1 and L2), and the dorsolateral branch of the third lumbar nerve (L3) supply sensory and motor innervation to the skin, fascia, muscles, and peritoneum of the flank. Regional analgesia of these nerves is the basis of the paravertebral block. For practical purposes with flank laparotomy, blocking of the dorsolateral branch of L3 is not generally considered necessary and may be contraindicated because if one has miscounted the vertebrae, one may actually block L4, which has nerve fibers running to the back legs.
Fig. 2-1 Inverted L Block.

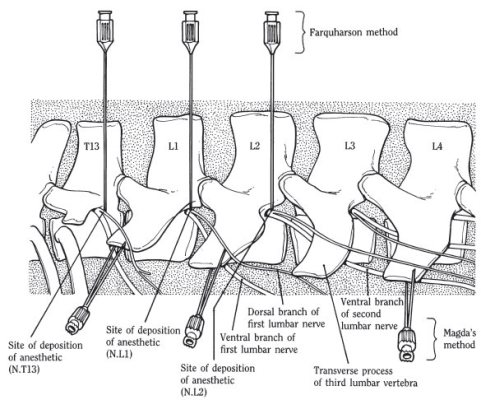
Various techniques for paravertebral block have been described. Walking the needle off the caudal edge of the transverse process, as illustrated in Figure 2-2, is most satisfactory. Anatomically, the nerve is most localized at its intervertebral foramen. By walking the needle off the caudal edge of the transverse process, one can deposit the analgesic solution close to the foramen; therefore, one has to block only a single site rather than the dorsal and ventral branches individually. The transverse processes are used as landmarks. Remembering that the transverse processes slope forward, the transverse process of L1 is used as a landmark to block T13, and the transverse processes of L2 and L3 are similarly used to locate nerves L1 and L2, respectively. When the transverse process has been located, a line is drawn from its cranial edge to the dorsal midline. The site for injection is 3 to 4 cm from the midline (Figure 2-2). The transverse process of L1 is difficult to locate in fat animals, in which case the site is estimated relative to the distance between the processes of L2 and L3. Local blebs are placed, and a 1-in, 16-gauge needle is inserted to act as a trocar in placing a 10-cm, 20-gauge needle. This second needle is inserted perpendicularly until the transverse process is encountered. The needle is then walked off the caudal border of the transverse process and advanced 0.75 cm; 10 ml of local analgesic solution are placed at each site. The incision site should be tested with a needle, and if the block has been properly placed, it will be effective almost immediately. In testing the block, one must remember that the distribution of the nerves is such that T13 innervates the ventral flank area, whereas L2 innervates the area close to the transverse processes.
Fig. 2-2. Paravertebral block.
A temporary lateral deviation of the spine due to muscle paralysis is observed in association with paravertebral analgesia.
Another technique favored by some surgeons is that developed by Magda and modified by Cakala.12 It uses a lateral approach to the nerves and would be more accurately described as a paralumbar rather than a paravertebral technique. The branches of T13, L1, and L2 are blocked close to the ends of the first, second, and fourth transverse processes, respectively, as illustrated in Figure 2-2. The skin is clipped and prepared at the ends of the first, second, and fourth lumbar transverse processes. An 18-gauge needle is inserted under each transverse process toward the midline, and 10 ml of solution are injected. The needle is then withdrawn a short distance and is redirected craniad and caudad while more solution is injected. In this fashion, a diffuse region ventral to the transverse process is infiltrated to block the ventral branch of the nerve. The needle is then redirected slightly dorsal and caudal to the transverse process to block the dorsolateral branches of the nerves. About 25 ml of solution are used for each site. Because the Cakala paralumbar technique does not paralyze the lumbar muscles, lateral deviation of the spine does not occur.
The technique for paravertebral nerve block is the same in sheep and goats as it is in cattle. Up to 5ml of 1 or 2% lidocaine is recommended for each of the injection sites.34 The total dose should not exceed 6 mg/kg, and onset may occur in as little as 5 minutes. Lidocaine with epinephrine may be used to increase the duration of analgesia to an hour or longer.34
Epidural analgesia is used frequently in large animal surgery for standing procedures in cattle and horses, cesarean sections in swine, urogenital surgery in goats, and postoperative analgesia. Sheep can be easily handled and may require only local analgesia and physical restraint for some procedures. On the other hand, goats have a low pain threshold and require analgesia and sedation. The technique for epidural injection is basically the same among small ruminants, cattle, and horses. Swine are more easily injected in the lumbrosacral space, however, than other species.
This technique consists of the deposition of local analgesic solution between the dura mater and periosteum of the spinal canal (epidural space), which in turn desensitizes the caudal nerve roots after they emerge from the dura. The degree of paralysis that is achieved depends chiefly on the volume of solution injected and on the concentration and diffusibility of the analgesic agent. The rate of absorption of local analgesic agent from the epidural space may contribute to the analgesic effect.
Epidural analgesia can be classified into cranial (high) or caudal (low), according to the area of spread of the analgesic solution and the extent of the area in which sensory and motor paralysis develops. Caudal epidural anesthesia implies that motor control of the hindlegs is not affected. Sensory innervation is lost from the anus, vulva, perineum, and caudal aspects of the thighs. The anal sphincter relaxes, and the posterior part of the rectum balloons. Tenesmus is relieved and obstetric straining is prevented. Caudal epidural anesthesia is inexpensive and routinely used in ruminants and horses.
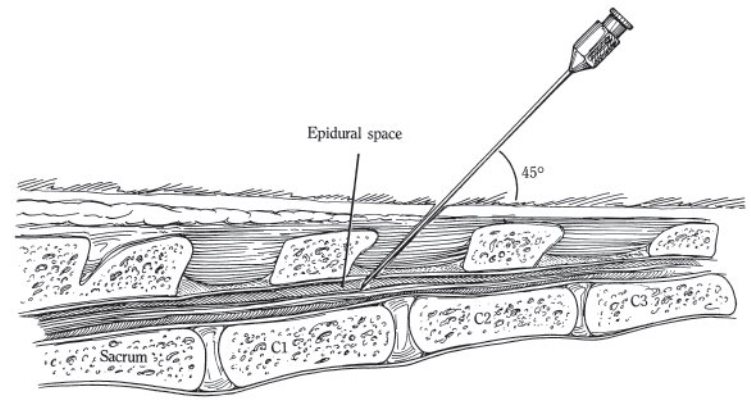
The injection site for caudal epidural analgesia is the same among ruminants and horses. The injection of the analgesic agent may be made between the first and second coccygeal vertebrae or in the sacrococcygeal space, although the former site is preferable because it is a larger space and is more easily detected, especially in fat animals. This site is 1 to 2 inches cranial to the long tail hairs in the horse. To locate the space, the tail is grasped and is moved up and down; the first obvious articulation caudal to the sacrum is the first intercoccygeal space. After clipping and skin preparation, a skin bleb is made with 2% lidocaine using a 2.5-cm, 25-gauge needle, to facilitate needle placement. An 18-gauge, 3- to 5-cm needle (or a spinal needle) is introduced through the center of the space on the midline at a 45° angle in the ox until its point hits the floor of the spinal canal (Figure 2-3). In the horse, this needle may be inserted at an angle of 30° from a perpendicular line through the vertebrae, or at an angle of 60° (as illustrated later in Figure 2-4). The needle is then retracted slightly to ensure that the end is not embedded in the intervertebral disc. If the needle is correctly placed in the epidural space, there should be no resistance to injection. In addition, one should make sure that the bevel of the needle is pointed forward, rather than to one side, to obtain even anesthesia.
In cattle and small ruminants, 2% lidocaine may be used for epidural anesthesia (doses shown in Table 2-1). Injections of 2 ml of 2% lidocaine can be used in the sacrococcygeal space of sheep and goats to provide caudal epidural analgesia for obstetric procedures.34,78 To achieve epidural analgesia for perineal and hindlimb surgical procedures in small ruminants, a lower dose of lidocaine is used (1 ml/7 kg). The total volume of lidocaine should not exceed 3 ml in sheep and goats and 10 ml in cattle to avoid hindlimb uncoordination and recumbency.78 Xylazine and 2% lidocaine are now more frequently used in cattle to achieve a longer duration of analgesia and quick onset.31
Local anesthetics are not as frequently used alone for caudal epidural anesthesia in horses; their onset of analgesia (about 20 minutes) is much slower than in cattle and the duration is relatively short (87.2 ± 7.5 min).32,34 For this reason, α2 agonists such as detomidine, xylazine, and medetomidine, are commonly used in combination with local anesthetics to increase the duration of analgesia and decrease ataxia.7,71 It is recommended that regardless of the drug used, the dose should not exceed 10 ml in horses to avoid hindlimb ataxia. Alternative anesthetic combinations are shown in Table 2-2.
Fig. 2-3. Bovine epidural anesthesia
Cranial epidural injection is considered contraindicated in horses but has some uses in other species. For example, this technique may be used to provide 2 to 4 hours of analgesia in cattle for laparotomy, pelvic limb surgery, or udder amputation. A higher dose of local anesthetic [1 ml/10 lb (3.73 kg) body weight] is used; the animal will go down and should be maintained in sternal recumbency for 10 to 15 minutes to ensure the even distribution of the analgesic solution. When inducing cranial epidural analgesia in cattle, the possible development of hypotension must be considered. No signs of hypotension have been observed using volumes of 100 to 150 ml of 2% lidocaine, but they have been recorded with volumes of 150 to 200 ml.33±
Continuous caudal epidural anesthesia using a commercial epidural catheter kit (Continuous Epidural Tray, American Hospital Supply, McGraw Park, IL.) is also used in horses and in some instances, food animals, for repeated epidural delivery of analgesics and postoperative pain relief.28,55,71 The kit contains a Huber-point directional needle with stylet (Tuohy spinal needle) inserted through a pilot hole at 45° to the horizontal until one encounters an abrupt reduction in resistance. The catheter is then inserted through the needle, it is advanced 2.5 to 4 cm beyond the end of the needle, and the needle is withdrawn. Combinations of either a local anesthetic or alpha-2 adrenergic agonist and morphine administered in the caudal epidural space have been shown to have useful clinical applications for postoperative and long-term pain relief in both humans and animals. Preoperative epidural administration of detomidine (30 µg/kg) and morphine (0.2 mg/kg) provides effective, long-lasting pain relief and decreases postoperative lameness in horses that undergo bilateral stifle arthroscopy.23
Epidural analgesia has been used in both young and adult pigs and, in particular, for cesarean section in the sow. In this instance, cranial (high) epidural analgesia has been used to effect both immobilization and analgesia without fetal depression. Cranial, rather than caudal, epidural analgesia is commonly performed.
Table 2-3