
Contents
Contributor List
Preface
About the Companion Website
SECTION 1: Introduction
CHAPTER 1: Percutaneous Renal Access: A Historical Perspective
Introduction
History
Percutaneous nephrolithotomy
Percutaneous transitional cell carcinoma resection
Percutaneous endopyelotomy
Other applications
Conclusion
References
CHAPTER 2: Interventional Imaging and Radiation Safety
Introduction
Basic radiation physics
Percutaneous renal surgery for stones
Interventional imaging and radiation safety for upper tract transitional cell carcinoma
Interventional imaging and radiation safety for percutaneous renal mass ablation
Conclusion
References
SECTION 2: Percutaneous Management of Large Renal Calculi (Percutaneous Nephrolithotomy)
CHAPTER 3: Epidemiology of Large Renal Stones and Utilization Patterns of Percutaneous Nephrolithotomy
Introduction
The large renal stone
Trends in percutaneous nephrolithotomy utilization
Conclusion
References
CHAPTER 4: Evolution of Evidence-Based Outcomes for Percutaneous Nephrolithotomy
Introduction
Choice of treatment
Surgical planning
Postoperative considerations
References
CHAPTER 5: Patient Selection and Informed Consent
Patient selection
Informed consent
Conclusion
References
CHAPTER 6: Instrumentation and Surgical Technique: Percutaneous Access
Informed consent
Preoperative preparation
Patient positioning
Instrumentation
Step-by-step technique
Intraoperative trouble-shooting
Postoperative follow-up
References
CHAPTER 7: Instrumentation and Surgical Technique: Tract Dilation and Endoscopes
Upper tract dilation
Endoscopes
Conclusion
References
CHAPTER 8: Instrumentation and Surgical Technique: Intracorporeal Lithotrites
Introduction
Pneumatic lithotripters
Ultrasonic lithotripters
Dual ultrasonic and ballistic lithotripters
Electrohydraulic lithotripter
Holmium:YAG laser
Intraoperative trouble-shooting for rigid intracorporeal lithotripters
Conclusion
References
CHAPTER 9: Instrumentation and Surgical Technique: Step-by-Step Percutaneous Nephrolithotomy: Prone
Introduction
Informed consent
Preoperative preparation
Patient positioning
Instruments
Step-by-step technique
Intraoperative tips/trouble-shooting
Postoperative care
Conclusions
References
CHAPTER 10: Instrumentation and Surgical Technique: Step-by-Step Percutaneous Nephrolithotomy: Supine
Introduction
Indications for supine percutaneous nephrolithotomy
Informed consent
Instrumentation
Technique
Visceral injury in supine percutaneous nephrolithotomy
Supine percutaneous nephrolithotomy in special situations
Outcome for supine percutaneous nephrolithotomy
Conclusion
References
CHAPTER 11: Instrumentation and Surgical Technique: Step-by-Step Percutaneous Nephrolithotomy: Prone-Flexed/Lateral
Patient positioning
Instrumentation
Step-by-step technique
Intraoperative trouble-shooting
References
CHAPTER 12: Instrumentation and Surgical Technique: Step-by-Step Percutaneous Nephrolithotomy: Endoscopic Guidance
Evolution of retrograde percutaneous access
Technique
Role in urological practice
Conclusion
References
CHAPTER 13: Instrumentation and Surgical Technique: Step-by-Step Percutaneous Nephrolithotomy: Mini-Percutaneous Nephrolithotomy
Introduction
Informed consent
Preoperative preparation
Patient positioning
Instrumentation
Step-by-step technique
Intraoperative trouble-shooting
Postoperative follow-up
References
CHAPTER 14: Instrumentation and Surgical Technique: Step-by-Step Percutaneous Nephrolithotomy: Multiple Access
Introduction
Patient preparation
Informed consent
Instrumentation
Anesthesia
Step-by-step technique
Intraoperative trouble-shooting
Follow-up
References
CHAPTER 15: Instrumentation and Surgical Technique: Step-by-Step Percutaneous Nephrolithotomy: Tube or Tubeless Percutaneous Nephrolithotomy
Introduction
Definition of tubeless percutaneous nephrolithotomy
Advantages of tubeless versus standard percutaneous nephrolithotomy
Use of hemostatic agents in tubeless percutaneous nephrolithotomy
Supracostal access
Special considerations
Informed consent
Preoperative preparation
Patient positioning
Instrumentation
Step-by-step technique
Intraoperative trouble-shooting
Postoperative follow-up
Postoperative trouble-shooting/auxiliary procedures after tubeless percutaneous nephrolithotomy
Conclusion and recommendations
References
CHAPTER 16: Instrumentation and Surgical Technique: Postoperative Imaging Following Percutaneous Nephrolithotomy
Introduction
The importance of residual fragments
Diagnosis of residual fragments
Evaluation of hydrothorax
Additional considerations
References
CHAPTER 17: Instrumentation and Surgical Technique: Step-by-Step Antegrade Ureteric Stenting
Principles and prerequisites
Patient preparation
Equipment
Patient position
Analgesia and sedation
Technique
Rendezvous stenting
Special situations
Pitfalls and dangers
Conclusion
References
SECTION 3: Percutaneous Management of Transitional Cell Cancer (Percutaneous Resection of Tumor)
CHAPTER 18: Epidemiology of Disease (Upper Tract Transitional Cell Cancer)
Overall incidence and trend
Tumor location and stage at presentation
Age, sex, and race
Smoking and occupational exposure
Analgesic abuse
Other environmental risk factors
Hereditary cases of upper tract transitional cell carcinoma
Metachronous upper tract transitional cell carcinoma
Metachronous bladder transitional cell carcinoma
Prognosis
References
CHAPTER 19: Evidence-Based Outcomes for Percutaneous Management of Upper Tract Urothelial Carcinoma
References
CHAPTER 20: Patient Selection and Informed Consent
Introduction
Patient selection
Informed consent
Conclusion
References
CHAPTER 21: Percutaneous Treatment of Upper Tract Urothelial Carcinoma
Introduction
Indications
Techniques
Results
Conclusion
References
SECTION 4: Percutaneous Ablation of Renal Cell Cancer (Thermal and Nonthermal)
CHAPTER 22: Epidemiology and Biology of Small Renal Masses
Epidemiology of small renal masses
Tumor biology and natural history of small renal masses
Renal mass sampling
Functional impact of small renal mass treatment
Management of small renal masses and role of thermal ablation (Table 22.2)
Conclusion
References
CHAPTER 23: Evolution of Evidence-Based Outcomes for Percutaneous Management
Introduction
Oncological outcomes
Complications
Preservation of renal function
Length of hospital stay and postoperative convalescence
Cost-effectiveness
Alternative techniques
Conclusion
References
CHAPTER 24: Patient Selection and Informed Consent
Introduction
Patient selection
Informed consent
References
CHAPTER 25: Instrumentation and Technique: Cryotherapy
Introduction
Patient selection
Informed consent
Preoperative patient preparation
Principles of ablation
Patient positioning
Instrumentation
Step-by-step technique
Management of complications
Postoperative follow-up
Conclusion
References
CHAPTER 26: Instrumentation and Technique: Hyperthermal Ablation: Radiofrequency and Microwave Ablation
Introduction
History
How radiofrequency ablation works (Figure 26.2)
Radiofrequency blation
Microwave ablation
Future outlook
Conclusion
References
CHAPTER 27: Instrumentation and Technique: High-Intensity Focused Ultrasound
Introduction
Informed consent
Preoperative preparation
Patient positioning
Instrumentation
Step-by-step technique
Intraoperative trouble-shooting
Postoperative follow-up
Conclusion
References
CHAPTER 28: Instrumentation and Technique: Laser
Introduction
Laser technology for renal ablation
Experimental studies
Clinical studies
Conclusion
References
CHAPTER 29: Instrumentation and Technique: Irreversible Electroporation
Background and preclinical data
Clinical data
General surgical considerations
Instrumentation and technology
Informed consent
Positioning and step-by-step technique
Postoperative follow-up
References
CHAPTER 30: Instrumentation and Techniques in Renal Radiosurgery
Informed consent
Preoperative preparation
Patient positioning
Instrumentation
Step-by-step technique (Video Clip 30.1)
Intraoperative troubleshooting
Posttreatment follow-up
References
CHAPTER 31: Instrumentation and Technique: Renal Histotripsy
Controlled acoustic cavitation
Tissue homogenization
Ultrasound feedback
Histotripsy dose–bioeffect relationship
Histotripsy thresholds and renal structures
Hemostasis with histotripsy
Chronic histotripsy effects
Histotripsy of malignant tissue
Conclusion
References
Index
To our parents:
Uma and Trilok Monga
Snehalata and Murali Rane

This edition first published 2014 © 2014 by John Wiley & Sons, Ltd
Registered Office
John Wiley & Sons, Ltd, The Atrium, Southern Gate, Chichester, West Sussex, PO19 8SQ, UK
Editorial Offices
9600 Garsington Road, Oxford, OX4 2DQ, UK
The Atrium, Southern Gate, Chichester, West Sussex, PO19 8SQ, UK
111 River Street, Hoboken, NJ 07030-5774, USA
For details of our global editorial offices, for customer services and for information about how to apply for permission to reuse the copyright material in this book please see our website at www.wiley.com/wiley-blackwell
The right of the author to be identified as the author of this work has been asserted in accordance with the UK Copyright, Designs and Patents Act 1988.
All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, except as permitted by the UK Copyright, Designs and Patents Act 1988, without the prior permission of the publisher.
Designations used by companies to distinguish their products are often claimed as trademarks. All brand names and product names used in this book are trade names, service marks, trademarks or registered trademarks of their respective owners. The publisher is not associated with any product or vendor mentioned in this book. It is sold on the understanding that the publisher is not engaged in rendering professional services. If professional advice or other expert assistance is required, the services of a competent professional should be sought.
The contents of this work are intended to further general scientific research, understanding, and discussion only and are not intended and should not be relied upon as recommending or promoting a specific method, diagnosis, or treatment by health science practitioners for any particular patient. The publisher and the author make no representations or warranties with respect to the accuracy or completeness of the contents of this work and specifically disclaim all warranties, including without limitation any implied warranties of fitness for a particular purpose. In view of ongoing research, equipment modifications, changes in governmental regulations, and the constant flow of information relating to the use of medicines, equipment, and devices, the reader is urged to review and evaluate the information provided in the package insert or instructions for each medicine, equipment, or device for, among other things, any changes in the instructions or indication of usage and for added warnings and precautions. Readers should consult with a specialist where appropriate. The fact that an organization or Website is referred to in this work as a citation and/or a potential source of further information does not mean that the author or the publisher endorses the information the organization or Website may provide or recommendations it may make. Further, readers should be aware that Internet Websites listed in this work may have changed or disappeared between when this work was written and when it is read. No warranty may be created or extended by any promotional statements for this work. Neither the publisher nor the author shall be liable for any damages arising herefrom.
Library of Congress Cataloging-in-Publication Data
Percutaneous renal surgery / [edited by] Manoj Monga, Abhay Rane.
p. ; cm.
Includes bibliographical references and index.
ISBN 978-1-118-27873-4 (hardback : alk. paper) – ISBN 978-1-118-67090-3 – ISBN 978-1-118-67092-7 (pub) – ISBN 978-1-118-67093-4 (pdf) – ISBN 978-1-118-67095-8 (mobi)
I. Monga, Manoj, editor of compilation. II. Ran?, Abhay, editor of compilation.
[DNLM: 1. Kidney Diseases–surgery. 2. Kidney–surgery. 3. Nephrostomy, Percutaneous–methods. 4. Urologic Surgical Procedures–methods. WJ 368]
RD575
617.4′61059–dc23
2013007101
A catalogue record for this book is available from the British Library.
Wiley also publishes its books in a variety of electronic formats. Some content that appears in print may not be available in electronic books.
Cover image: ©John Wiley & Sons Ltd.
Cover design by OptaDesign.co.uk
J. Kyle Anderson MD
Associate Professor
Department of Urology
University of Minnesota
Minneapolis, MN, USA
Kirk M. Anderson MD
Resident
Loma Linda University School of Medicine
Loma Linda, CA, USA
Don C. Arnold II MD
Minimally Invasive Urologic Surgery Fellow
Loma Linda University School of Medicine
Loma Linda, CA, USA
Dean G. Assimos MD
Professor and Chairman
Department of Urology
University of Alabama Birmingham Medical Center
Birmingham, AL, USA
Aditya Bagrodia MD
Resident, Department of Urology
Jane and Charles Y. C. Pak Center for Mineral Metabolism
The University of Texas Southwestern Medical Center
Dallas, TX, USA
D. Duane Baldwin MD
Professor of Urology
Endourology Program Director
Director of Urologic Research
Loma Linda University School of Medicine
Loma Linda, CA, USA
Naeem Bhojani MD
Endourology Fellow
Indiana University School of Medicine
Indianapolis, IN, USA
Jason R. Bylund MD
Endourology Fellow
Division of Urology
University of Kentucky
Lexington, KY, USA
Jeffrey A. Cadeddu MD
Professor of Urology
The University of Texas Southwestern Medical Center
Dallas, TX, USA
Steven C. Campbell MD, PhD
Professor of Surgery
Center for Urologic Oncology
Glickman Urologic and Kidney Institute
Cleveland Clinic
Cleveland, OH, USA
Arturo Castro Jr MD
Research Fellow
Division of Endourology
Robotics, Laparoscopy and Minimally Invasive Surgery
Joint Bioengineering and Endourology Development Surgical Laboratory
Department of UrologyMiller School of Medicine, University of Miami
Miami, FL, USA
Doh Yoon Cha MD
Postdoctoral Clinical Fellow
Department of Urology
Columbia University School of Medicine
New York, NY, USA
Ben Chew MD, MSc, FRCSC
Assistant Professor
Department of Urologic Sciences
University of British Columbia
Vancouver, BC, Canada
Jane Cho MD
Resident
Department of Urology
University of California Irvine
Orange, CA, USA
Ralph V. Clayman MD
Professor
Department of Urology
Dean, School of Medicine University of California Irvine
Orange, CA, USA
Michael Conlin MD, FACS
Associate Professor
Department of Urology
Portland VA Medical Center
Oregon Health and Sciences University
Portland, OR, USA
David Cranston MB, ChB, MA, DPhil, FRCS
Consultant Urological Surgeon
Oxford University Hospitals NHS Trust;Clinical Director, Oxford HIFU Unit
Senior Lecturer, Nuffield Department of Surgical Sciences
University of Oxford
Oxford, UK
Paul L. Crispen MD
Assistant Professor of Surgery
Division of Urology
University of Kentucky
Lexington, KY, USA
John Denstedt MD, FRCSC, FACS
Richard Ivey Professor and Chair/Chief
Department of Surgery
Schulich School of Medicine & Dentistry
The University of Western Ontario;
London Health Sciences Centre; St Joseph’s Health Care London
London, ON, Canada
Mahesh R. Desai MD, MS, FRCS
Medical Director
Muljibhai Patel Urological Hospital
Nadiad, India
Mihir Desai MD
Professor of Urology
Keck School of Medicine University of Southern California
Los Angeles, CA, USA
Steve Dong MD
Fellow in Advanced Endourology, Laparoscopy, and Robotic Surgery
Keck School of Medicine University of Southern California
Los Angeles, CA, USA
Amit Doshi MS(General Surgery)
Resident in Urology
Muljibhai Patel Urological Hospital
Nadiad, India
Matthew Dunn MD
Clinical Assistant Professor of UrologyDirector of Endourology and Stone Disease
Keck School of Medicine, University of Southern California
Los Angeles, CA, USA
Obi Ekwenna MD
Chief Resident in Urology
Division of Endourology
Robotics, Laparoscopy and Minimally Invasive Surgery
Joint Bioengineering and Endourology Development Surgical Laboratory
Department of Urology Miller School of Medicine, University of Miami
Miami, FL, USA
Stephen Faddegon MD
Fellow
Department of Urology
The University of Texas Southwestern Medical Center
Dallas, TX, USA
Nader Fahmy MD, PhD, FRCSC
Fellow in Endourology
Division of Urology, Department of Surgery
Schulisch School of Medicine & Dentistry
The University of Western Ontario;
London Health Sciences Centre; St Joseph’s Health Care London
London, ON, Canada
Kirsten Foell MD, FRCSC
Endourology and Minimally Invasive Surgery Fellow
St. Michael’s Hospital
University of Toronto
Toronto, ON, Canada
Arvind Ganpule MS, DNB(Urol)
Consultant Urologist
Muljibhai Patel Urological Hospital
Nadiad, India
Joseph Graversen MD
Minimal Invasive Urology Fellow
Department of Urology
University of California Irvine
Orange, CA, USA
Mantu Gupta MD
Associate Professor of Urology
Department of Urology
Columbia University School of Medicine
New York, NY, USA
R. John D’A. Honey MA, MB, BChir, FRCS(Eng), FRCSC
Professor of Surgery
Director of Endourology
St Michael’s Hospital
University of Toronto
Toronto, ON, Canada
Matthew R. Hotston MD, FRCS(Urol)
Consultant Urologist
Royal Cornwall Hospital
Truro, UK
Lawrence Jenkins MD
Resident
Division of Endourology
Robotics, Laparoscopy and Minimally Invasive Surgery
Joint Bioengineering and Endourology Development Surgical Laboratory
Department of Urology Miller School of Medicine, University of Miami
Miami, FL, USA
Jihad H. Kaouk MD
Zegarac-Pollock Professor of Surgery
Institute Vice Chair for Surgical Innovations
Director, Center for Laparoscopic and Robotic Surgery
Glickman Urological and Kidney Institute
Cleveland Clinic
Cleveland, OH, USA
Francis X. Keeley MD, FRCS(Urol)
Consultant Urologist
Bristol Urological Institute
Southmead Hospital
Bristol , UK
Farhan Khan MD
Resident
Department of Urology
University of California Irvine
Orange, CA, USA
Thomas Knoll MD, PhD, MSc
Head and Chairman
Department of Urology
Klinikum Sindelfingen-Böblingen
University of Tuebingen
Sindelfingen, Germany
Bodo E. Knudsen MD, FRCSC
Director, OSU Comprehensive Kidney Stone Program
Vice-Chair Clinical Operation
Assistant Professor, Department of Urology
Wexner Medical Center, The Ohio State Univeristy
Columbus, OH, USA
Ravi Kulkarni MS, FRCS
Consultant Urological Surgeon
Ashford and St Peter’s Hospitals
Chertsey, UK
Jaime Landman MD
Professor of Urology and Radiology and Chairman
Department of Urology
University of California Irvine
Orange, CA, USA
Jessica N. Lange MD
Urology Resident
Department of Urology
Wake Forest Baptist Health
Winston-Salem, NC, USA
Humberto Laydner MD
Center for Laparoscopic and Robotic Surgery
Glickman Urological and Kidney Institute
Cleveland Clinic
Cleveland, OH, USA
David A. Leavitt MD
Resident
Department of Urology
University of Minnesota
Minneapolis, MN, USA
Tom Leslie MB, ChB, DPhil, FRCS(Urol)
Clinical Lecturer in Urology
Nuffield Department of Surgical Sciences
University of Oxford
Oxford, UK
Raymond Leveillee MD
Chief
Division of Endourology
Robotics, Laparoscopy and Minimally Invasive Surgery
Joint Bioengineering and Endourology Development Surgical Laboratory
Department of UrologyMiller School of Medicine, University of Miami
Miami, FL, USA
James E. Lingeman MD
Professor of Urology
Indiana University School of Medicine
Indianapolis, IN, USA
Michael E. Lipkin MD
Assistant Professor of Urology
Division of Urologic Surgery
Comprehensive Kidney Stone Center
Duke University Medical Center
Durham, NC, USA
Michael A. Liss MD
Chief Resident
Department of Urology
University of California Irvine
Orange, CA, USA
Achim Lusch MD
Minimal Invasive Urology Fellow
Department of Urology
University of California Irvine
Orange, CA, USA
Sunil Mathur MD, FRCS(Urol)
Clinical Fellow in Urology
Bristol Urological Institute
Southmead Hospital
Bristol, UK
Matthew J. Maurice MD
Resident
Urologic Oncology and Minimally Invasive Therapies Center
Urology Institute
University Hospitals Case Medical Center
Cleveland, OH, USA
Michael J. Metcalfe BSc, MD
PGY-3 Resident
Department of Urologic Sciences
University of British Columbia
Vancouver, BC, Canada
Ross Moskowitz MD
Resident
Department of Urology
University of California Irvine
Orange, CA, USA
Patrick W. Mufarrij MD
Assistant Professor
Department of Urology
George Washington University Medical Center
Washington, DC, USA
Andreas Neisius MD
Fellow in Endourology
Division of Urologic Surgery
Kidney Stone Center, Division of Urologic Surgery
Duke University Medical Center
Durham, NC, USA;
Department of Urology
Johannes Gutenberg University
Mainz, Germany
Zeph Okeke MD
Assistant Professor of Urology, Attending Physician
Arthur D. Smith Institute for Urology
Hofstra University
North Shore Long Island Jewish School of Medicine
New Hyde Park, NY, USA
Michael Ordon MD, FRCSC
Assistant Clinical Professor
Department of Urology
University of California Irvine
Orange, CA, USA
Matthew J. O’Shaughnessy MD, PhD
Resident
Department of Urology
University of Minnesota
Minneapolis, MN, USA
Kenneth T. Pace MD, MSc, FRCSC
Head
Division of Urology, St Michael’s Hospital
Researcher, Keenan Research Centre Li Ka Shing Knowledge Institute;
Associate Professor, Department of Surgery
University of Toronto,
Toronto, ON, Canada
Abhishek P. Patel MD
Resident
Department of Urology
Wexner Medical Center, The Ohio State University
Columbus, OH, USA
Ryan Paterson MD, FRCSC
Assistant Professor
Department of Urologic Sciences
University of British Columbia
Vancouver, BC, Canada
Margaret S. Pearle MD, PhD
Professor of Urology and Internal Medicine
Jane and Charles Pak Center for Mineral Metabolism
The University of Texas Southwestern Medical Center
Dallas, TX, USA
Lee E. Ponsky MD, FACS
Associate Professor Urology
Leo and Charlotte Goldberg Chair in Advanced Surgical Therapies
Urologic Oncology and Minimally Invasive Therapies Center
Urology Institute
University Hospitals Case Medical Center
Cleveland, OH, USA
Glenn M. Preminger MD
James F. Glenn Professor and Chief of Urology
Comprehensive Kidney Stone Center, Division of Urologic Surgery
Duke University Medical Center
Durham, NC, USA
Robert W. Ritchie MD, PhD
Clinical Research Fellow in HIFU
Nuffield Department of Surgical Sciences
University of Oxford
Oxford, UK
William W. Roberts MD
Associate Professor of Urology and Biomedical Engineering
University of Michigan
Ann Arbor, MI, USA
Simpa S. Salami MD, MPH
Resident
The Arthur Smith Institute for Urology
Hofstra University School of Medicine
North Shore Long Island Jewish Health System
New Hyde Park, NY, USA
Nelson Salas PhD
Research Assistant Professor
Division of Endourology
Robotics, Laparoscopy and Minimally Invasive Surgery
Joint Bioengineering and Endourology Development Surgical Laboratory
Department of UrologyMiller School of Medicine, University of Miami
Miami, FL, USA
John Shields MD
Division of Endourology
Robotics, Laparoscopy and Minimally Invasive Surgery
Joint Bioengineering and Endourology Development Surgical Laboratory
Department of Urology
Miller School of Medicine, University of Miami
Miami, FL, USA
Arthur D. Smith MD, MB, BCh FCS(SA)
Professor of Urology
Hofstra University School of Medicine;
Chairman Emeritus
North Shore Long Island Jewish Medical Centre
New Hyde Park, NY, USA
Marshall L. Stoller MD
Professor and Vice Chair of Urology
Department of Urology
University of California
San Francisco, CA, USA
Stephen E. Strup MD
Chief
Division of Urology
University of Kentucky
Lexington, KY, USA
Mark Sullivan MBBS, BSc MD, FRCS(Urol)
Consultant Urological Surgeon and Honorary Senior Lecturer
Oxford University
Nuffield Department of Surgical Sciences
University of Oxford
Oxford, UK
Nicholas Tadros MD
Resident in Urology
Department of Urology
Portland VA Medical Center
Oregon Health and Sciences University
Portland, OR, USA
Eric R. Taylor MD
Resident
Division of Urology
Southern Illinois University School of Medicine
Springfield, IL, USA
Chad R. Tracy MD
Assistant Professor of UrologyDirector of Minimally Invasive Surgery
University of Iowa Hospitals and Clinics
Iowa City, IA, USA
Ramakrishna Venkatesh MD, FRCS (Urology)
Associate Professor
William Farish Professor of Surgery
Division of Urology
University of Kentucky
Lexington, KY, USA
Gino J. Vricella MD
Chief Resident
Urologic Oncology and Minimally Invasive Therapies Center
Urology Institute University Hospitals Case Medical CenterCleveland, OH, USA
Gunnar Wendt-Nordahl MD, PhD, FEBU
Vice-Chair
Department of Urology
Klinikum Sindelfingen-Böblingen
University of Tuebingen
Sindelfingen, Germany
As medical students, we learn that the kidney receives 20% of the cardiac output. The thought of making a 1 cm hole in the kidney and relying on the forces of nature for hemostasis is understandably met with trepidation. Yet, the foresight, innovation, and courage of our predecessors have paved our path towards minimally invasive percutaneous approaches to renal diseases.
In this book we explore both the novel developments in percutaneous renal surgery and percutaneous ablative techniques, recognizing that the overlap in anatomical considerations, radiological and surgical skill sets, instrumentation and technique present an opportunity for collaboration and synergy.
Manoj Monga
Abhay Rane

Percutaneous nephrolithotomy (PCNL) has evolved to become the preferred minimally invasive approach for treating large-burden renal stones. This approach has replaced open renal surgery for stones. It has also evolved into a treatment option for treating noninvasive urothelial tumors of the upper urinary tract. Current percutaneous access techniques involve fluoroscopic or ultrasound guidance with a small-gauge needle for initial access. In selected cases, computed tomography (CT) guidance or blind access by anatomical landmarks may be indicated. The complications of PCNL are minimal and the associated morbidity is far less than for open renal surgery.
In 1865 at the Great Ormond Street Hospital, a case report by Thomas Hillier of therapeutic percutaneous renal decompression in a 4-year-old boy with congenital obstruction of the ureteropelvic junction was the first such case of percutaneous nephrostomy [1]. Through the course of the following 5 years, he performed multiple nephrostomies to relieve the recurring abdominal distension from the obstructed kidney. However, there was no suitable trocar available with which to create a permanent nephrocutaneous fistula. The child subsequently died at the age of 8 after a febrile illness.
The history of modern percutaneous renal surgery began with the first image-guided renal biopsy performed in 1944 by Nils Alwall by means of needle aspiration using a simple radiograph and retrograde pyelogram to localize the kidney with the patient in the sitting position. The procedure was performed at the University of Lund, Sweden. While the initial procedure was performed in 1944, the experience was not published until 1952 [2]. Subsequent series of percutaneous renal biopsy had the patient positioned prone, and the kidneys were localized using landmark distances between the vertebral spinous processes and the 11th and 12th ribs, and palpation for kidney movement [3].
The next reported description of percutaneous renal access was in 1955. Goodwin, Casey, and Woolf presented their experience at Harbor General Hospital of the University of California, Los Angeles, in 16 patients with hydronephrosis managed with percutaneous nephrostomies for drainage. All cases were performed under local anesthesia [4]. This technique followed as a natural extension of their initial report of percutaneous antegrade pyelography [5]. The authors noted in their technique that the procedure should be limited to patients with severe hydronephrosis since it was easier to puncture a larger hydronephrotic sac. The punctures were made with 12–14 gauge needles. It is of interest to note that the authors fashioned polyethylene tubings with several additional lateral holes to increase urinary drainage, allowing 2–4 inches of that portion of the tubing to coil in the renal pelvis. These early modifications are now the standard design of pigtail nephrostomy tubes that are currently available.
In 1975, Stables published a case series in which he described a technique to convert a standard temporary percutaneous nephrostomy to prolonged or permanent nephrostomy drainage with Foley catheters [6]. This was thought to be of benefit in the management of obstructive nephropathy in cases where the primary lesion was not readily amenable to surgical repair. In a larger series and review of the literature, Stables described the application of the percutaneous nephrostomy in supravesical urinary obstruction, urinary fistulas, and renal calculi [7]. He reported a success rate of over 90% with percutaneous nephrostomies, with major complications limited to 4% and minor complications to 15%. This represented a significant advance because open nephrostomy had been associated with such complications as uremia, hemorrhage, infections, sepsis, and at times difficult access to the renal pelvis.
In another series, Hellsten et al. reported performing percutaneous nephrostomy in 32 patients. Of these, eight patients were for permanent drainage and 24 for temporary drainage. Malignant obstruction of the distal ureter was the most common indication [8]. Access to the renal pelvis was obtained with the aid of fluoroscopy and/or ultrasound. Change to larger catheters was achieved using the Seldinger technique. The most common complication reported was hemorrhage in five patients. Percutaneous nephrostomy has thus remained in use for temporary or permanent drainage of the urinary tract for various indications including infections, obstruction or neoplasm.
In 1976, Fernstrom and Johannson reported on the first percutaneous image-guided nephrolithotomy [9]. The tract was dilated coaxially with graded plastic dilators over the course of a few days. The tract was then used for renal manipulation using grasping tools and Dormia baskets after allowing the tract to mature. Following shortly thereafter in 1979, Smith et al. from the University of Minnesota reported on their experience [10]. By 1984, they reported results from their first 100 patients [11]. Interestingly, the complication rate decreased to 5% as their experience with the procedure grew, with a reported stone-free rate of 91% for the most recent patients in that series. One year later in 1985, the same group reported on a further 400 patients. This time, the stone-free rate had improved to 99% for patients with renal stones and 94.5% for ureteral stones [12]. Their results compare very favorably to stone-free and complication rates from more modern PCNL series.
In 1984, Orihuela, Crowley, and Smith from the Long Island Jewish Medical Center in New York extended the techniques for percutaneous renal access to the treatment of upper tract urothelial carcinomas. Two years later, they became the first to report their experience at the annual meeting of the American Urological Association in 1986 [13]. The initial series of patients was a highly selected group suitable for renal-sparing surgery for solitary kidney, bilateral synchronous disease, renal insufficiency, poor surgical risk for open surgery or biopsy evidence of a solitary low-grade superficial tumor. The authors’ technique involved an initial resection through a percutaneous nephrostomy, followed by a second-look procedure 2–28 days later to assess the completeness of the initial resection and to remove any residual tumors. Of note, with this initial series of patients, the authors used adjuvant topical therapy through the nephrostomy tube with mitomycin C and bacillus Calmette–Guerin (BCG). Subsequently, the same group published results on their experience with their first nine patients [14]. Other authors emulated the technique and reported similar success [15].
The percutaneous renal access technique was also adapted to treatment of the obstructed ureter. With percutaneous renal access, the ureteropelvic junction is easily accessible and made endoscopic incision feasible, avoiding the need for open surgery. In 1983, Whitfield et al. described a procedure of percutaneous incision of the ureteropelvic junction using a modification of the Davis intubated ureterostomy technique. The authors reported a success rate of 64% with their technique [16]. In 1984, Smith reported on the various adaptations of the nephrostomy tract to renal surgery [17]. He demonstrated that the nephrostomy tract permits antegrade insertion of ureteral stents, ureteral dilation, and insertion of ureteral catheters to which other instruments such as stone baskets, steel stylets, etc. could be attached, thus facilitating controlled stone manipulation, ureteral meatotomy, and retrograde stent insertion. He adapted the technique reported by Whitfield et al. and termed it endopyelotomy. In 1986, Badlani et al. reported on their initial experience in the treatment of ureteropelvic junction obstruction using this modified technique in 31 patients with a cold knife direct-vision urethrotome inserted through a percutaneous nephrostomy tract [18]. A success rate of 87.1% was reported by the authors. Notably, eight of these patients were undergoing endopyelotomy after previous failed open pyeloplasty.
The next application of the nephrostomy tract was the attempt to dissolve stones by chemolysis. The instillation of acetylcysteine together with sodium bicarbonate through a nephrostomy tube into the renal pelvis was highly effective for dissolving cystine stones. Subsequently, renacidin was used to dissolve struvite stones. Blaivas et al. attempted chemical dissolution of residual stone fragments in 12 instances via nephrostomy tube irrigation [19]. Solutions containing either hemiacidrin or sodium bicarbonate were used for struvite and uric acid stones respectively, with a 75% success rate (complete dissolution of stones) reported. Pfister and Dretler also reported a considerably higher success rate in the management of renal and ureteral calculi with chemolytic drug irrigation through a percutaneous nephrostomy catheter [20]. Struvite, apatite, and carbonate stones were dissolved with an acidic solution (hemiacidrin, Suby solution G) and cystine stones were dissolved with an alkaline agent (Tham-E, acetylcysteine). A success rate of 85% in more than 150 stones cases was reported. This was particularly advantageous in medical conditions (cardiac, metabolic) where oral alkalinization with sodium bicarbonate or potassium citrate may be contraindicated. However, it took about 3–4 weeks of continuous irrigation to dissolve a stone. Chemolysis has since fallen out of favor due to the excellent outcomes from percutaneous nephrolithotomy.
The evolution of percutaneous renal access became the highway. The early innovations in minimally invasive urological surgery have evolved into what has today become the standard of care for many urological diseases. Those experiences propelled minimally invasive urology in great leaps and bounds. Not long after the early days of percutaneous renal surgery, in 1991 Ralph Clayman, one of the early pioneers, performed the first laparoscopic nephrectomy [21]. That endeavor has driven the evolution of urological surgery to a craft that is accomplished primarily through minimally invasive techniques.
1 Bloom DA, Morgan RJ, Scardino PL. Thomas Hillier and percutaneous nephrostomy. Urology 1989;33(4):346–50.
2 Alwall N. Aspiration biopsy of the kidney, including i.a. a report of a case of amyloidosis diagnosed through aspiration biopsy of the kidney in 1944 and investigated at an autopsy in 1950. Acta Med Scand 1952;143(6):430–5.
3 Kark RM, Muehrcke RC. Biopsy of kidney in prone position. Lancet 1954;266(6821):1047–9.
4 Goodwin WE, Casey WC, Woolf W. Percutaneous trocar (needle) nephrostomy in hydronephrosis. JAMA 1955;157(11):891–4.
5 Casey WC, Goodwin WE. Percutaneous antegrade pyelography and hydronephrosis; direct, intrapelvic injection of urographic contrast material to secure a pyeloureterogram after percutaneous needle puncture and aspiration of hydronephrosis. J Urol 1955;74(1):164–73.
6 Stables DP, Holt SA, Sheridan HM, Donohue RE. Permanent nephrostomy via percutaneous puncture. J Urol 1975;114(5):684–7.
7 Stables DP, Ginsberg NJ, Johnson ML. Percutaneous nephrostomy: a series and review of the literature. Am J Roentgenol 1978;130(1):75–82.
8 Hellsten S, Hildell J, Link D, Ulmsten U. Percutaneous nephrostomy. Aspects on applications and technique. Eur Urol 1978;4(4):282–7.
9 Fernstrom I, Johansson B. Percutaneous pyelolithotomy. A new extraction technique. Scand J Urol Nephrol 1976;10(3):257–9.
10 Smith AD, Reinke DB, Miller RP, Lange PH. Percutaneous nephrostomy in the management of ureteral and renal calculi. Radiology 1979;133(1):49–54.
11 Clayman RV, Surya V, Miller RP, et al. Percutaneous nephrolithotomy: extraction of renal and ureteral calculi from 100 patients. J Urol 1984;131(5):868–71.
12 Reddy PK, Hulbert JC, Lange PH, et al. Percutaneous removal of renal and ureteral calculi: experience with 400 cases. J Urol 1985;134(4):662–5.
13 Orihuela E, Crowley AR, Smith AD. Percutaneous management of renal pelvic transitional cell carcinoma (TCCA). J Urol 1986;135:164A.
14 Smith AD, Orihuela E, Crowley AR. Percutaneous management of renal pelvic tumors: a treatment option in selected cases. J Urol 1987;137(5):852–6.
15 Clark PE, Streem SB, Geisinger MA. 13-year experience with percutaneous management of upper tract transitional cell carcinoma. J Urol 1999;161(3):772–5; discussion 5–6.
16 Whitfield HN, Mills V, Miller RA, Wickham JEA. Percutaneous pyelolysis: an alternative to pyeloplasty. Br J Urol 1983:55(suppl):93–6.
17 Smith AD. Percutaneous ureteral surgery and stenting. Urology 1984;23(5 spec no):37–42.
18 Badlani G, Eshghi M, Smith AD. Percutaneous surgery for ureteropelvic junction obstruction (endopyelotomy): technique and early results. J Urol 1986;135(1):26–8.
19 Blaivas JG, Pais VM, Spellman RM. Chemolysis of residual stone fragments after extensive surgery for staghorn calculi. Urology 1975;6(6):680–6.
20 Pfister RC, Dretler SP. Percutaneous chemolysis of renal calculi. Urol Radiol 1984;6(2):138–43.
21 Clayman RV, Kavoussi LR, Soper NJ, et al. Laparoscopic nephrectomy. N Engl J Med 1991;324(19):1370–1.
Medical imaging plays an essential role during the diagnosis, treatment, and follow-up of patients undergoing percutaneous renal surgery. As minimally invasive surgery has employed smaller and less invasive techniques for renal surgery, surgeons have increasingly relied upon interventional imaging to provide the information regarding anatomical relationships and pathology which previously could be directly seen or felt during open surgery. These imaging modalities have allowed surgeons to gain percutaneous access to the kidney for stone treatment, treat upper tract transitional cell carcinoma (TCC) in an endoscopic manner and perform percutaneous renal ablative surgery for small renal tumors. However, this increased reliance upon medical imaging during diagnosis, treatment, and follow-up has increased the radiation exposure received by patients. It is important that the surgeon performing minimally invasive renal surgery is facile with interventional imaging, knowledgeable regarding basic radiation physics and fully appreciates the differences between imaging modalities. Finally, the surgeon must use this knowledge to select diagnostic, therapeutic and follow-up imaging in a manner that will provide the optimal outcome and patient safety while minimizing the radiation exposure to the patient, surgeon, and staff.
A fundamental understanding of the biological effects of radiation in the patient is essential in order to optimally utilize diagnostic and therapeutic imaging. The term “absorbed dose” refers to the amount of energy deposited per unit mass and is a way to determine the probability of biological effect (Box 2.1). Absorbed dose is measured in units of gray (Gy) or milligray (mGy). One gray is equal to 1 joule per kilogram of tissue (J/kg). Entrance skin dose refers specifically to the measure of radiation dose absorbed by the skin where the x-ray beam enters the patient. Finally, organ dose describes the amount of radiation to the organs of a patient [1, 2].
The two types of biological effects observed in patients following radiation exposure include immediate deterministic effects and delayed stochastic effects. Deterministic effects typically have a short latency period and are characterized by nonlinear dose–responses with a threshold dose (> 0.1 Gy) [3]. At lower doses, these deterministic effects (i.e. erythema and epilation) will completely resolve but above 10 Gy permanent damage may result [4].
The stochastic effects of radiation include the development of secondary malignancies. Unlike deterministic effects, there are no data to support a threshold below which stochastic effects will not occur [3]. The development of secondary malignancies is thought to be due to misrepair of damaged DNA that results in a genetic transformation. This damage is directly correlated with the total radiation absorbed by organs and tissues [5]. Since intentionally exposing patients to high levels of radiation would be unethical, much of our understanding of radiation’s stochastic effects is inferred from the effects observed in atomic bomb survivors. However, since atomic bomb survivors also received neutrons, protons, and other radioactive materials for which the biological effects are not as well characterized, this may not be the most accurate comparison [6,7].
| Radiation conversions | |
| 1 mGy | 100 mrad |
| 1 mSv | 100 mrem |
| 1 mGy* | 1 mSv* |
| 1 rad* | 1 rem* |
| 1 Gy | 100 roentgen |
| 10 mSv | 1/1000 develop cancer; 1/2000 fatal cancer [107] |
| 100 mSv | 1% increase in cancer in a population [108] |
| 1 Sv | Onset of early radiation effects [109] |
| 2 Sv | Threshold for early death [109] |
| 4 Sv | 50% chance for survival [109] |
| Environmental exposures | |
| Natural background radiation per year | 2.72 mSv [110] |
| Cosmic radiation per year | 0.28 mSv [110] |
| Radiation from airport scanners (50 kVp) | 0.9 uSv [111] |
| Airplane flight from New York to London | 0.1 mSv [112] |
| Within 3 km of Hiroshima detonation | 50–100 mSv [112] |
| 237 onsite Chernobyl workers at meltdown | 1–16 Sv [113] |
| Medical imaging exposures | |
| KUB | 0.7 mSv [47] |
| Chest x-ray 2 view | 0.05–0.24 [47] |
| Voiding cystourethrogram | 0.2–8.5 mSv [114] |
| Intravenous pyelogram | 3–9 mSv [56,115] |
| Noncontrast CT abdomen or pelvis (1 phase) | 10 mSv [112] |
| CT urogram | 14.8–36.1 mSv [56,116] |
| Nuclear renal scan DTPA | 1.8 mSv [47] |
| Nuclear renal scan MAG 3 | 2.6 mSv [47] |
| Nuclear renal scan DMSA | 3.3 mSv [47] |
| Bone scan | 6.3 mSv [47] |
| PET scan | 14.1 mSv [47] |
| Low-dose CT abdomen and pelvis | 2.1 mSv [117] |
| Ultra low-dose CT abdomen and pelvis | 0.95 mSv [112] |
| Percutaneous cryoablation | 120 mSv [118] |
| Exposure recommendations | |
| Maximum occupational radiation exposure | 20 mSv/year averaged over 5 years with no more than 50 mSv in any one year [119] |
| Allowable exposure to the lens of the eye/yr | 150 mSv [119] |
| Hands and feet/yr | 500 mSv [119] |
At the present time the linear no-threshold model is felt to best represent the risk for stochastic injury following radiation damage. It has been documented that solid cancer rates will increase by 35% per Gy for men and 58% per Gy for women after exposure at age 30 if they live to 70 years of age [8]. The likelihood that exposing patients to ionizing radiation will result in cancer is dependent upon how much radiation they absorb, the type of radiation they are exposed to, and the sensitivity of the organ exposed.
Humans receive radiation from a variety of natural and iatrogenic sources (see Box 2.1). During the diagnosis, treatment, and follow-up of patients undergoing percutaneous renal surgery, patients may receive substantial radiation exposure. Most of this imaging is essential to allow safe and effective patient treatment. However, it has been estimated that of the 80 million computed tomography (CT) scans performed annually, 20–40% may be unnecessary [9]. Medical imaging currently contributes to approximately 50% of overall radiation exposure in the United States compared to 15% in 1980 [9]. It was recently estimated that 29,000 tumors may result from the 70 million CT scans performed in the United States in 2007 alone and this may account for 2% of US cancers [10]. In 2010, the Food and Drug Administration (FDA) issued a White Paper calling for a reduction in the radiation exposure received by patients during medical imaging and specifically recommended reductions in radiation from CT, fluoroscopy, and nuclear medicine imaging [11].
The treatment of large renal stones with percutaneous nephrostolithotomy (PCNL) begins by obtaining an appropriate characterization of the preoperative stone volume and location, anatomical relationships and at least indirect information to suggest the presence of adequate renal function. Although a plain abdominal radiograph (KUB), intravenous pyelogram (IVP), nuclear renography, and renal ultrasound (US) are sometimes used, CT imaging is the most commonly employed modality in the evaluation of staghorn calculi. CT acquires images rapidly, is nearly universally available, and provides important anatomical relationships. CT imaging can also create three-dimensional (3D) reconstructions to assist in tract site planning [12], although the benefits of the 3D reconstructions are not uniformly accepted [13]. Furthermore, in some patients with complicated anatomy, the nephrostomy tube may have to be placed using CT guidance [14].
Figure 2.1 Plain abdominal x-ray demonstrating full bilateral staghorn calculi.
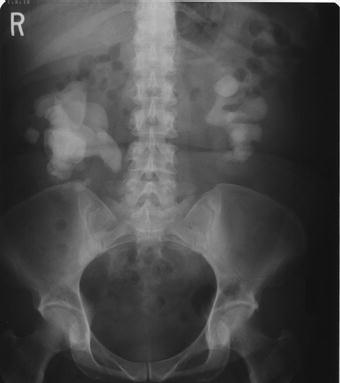
Although magnetic resonance imaging (MRI) provides excellent soft tissue imaging, its use in the evaluation of staghorn calculi has been limited due to poor visualization of stones, high cost, long acquisition time, and degradation with motion artifact [15,16]. Ultrasound is able to accurately detect renal calculi, determine parenchymal thickness and access for hydronephrosis without ionizing radiation. A KUB is also routinely performed to give an overview of the stone size and location, and to determine whether the stone is radiopaque (Figure 2.1). A nuclear renogram may be helpful to access renal function in staghorn patients, particularly in those with long-standing hydronephrosis, parenchymal thinning or prior interventions [17].
The most common imaging modality employed intraoperatively in the treatment of staghorn calculi is fluoroscopy. Appropriate utilization of fluoroscopy during PCNL provides an important understanding of anatomical and spatial relationships that leads to a decrease in the complexity and improved procedure safety. The cinematic images are particularly helpful for advancing the guidewire past the stone into the ureter prior to tract dilation [18].
Computed tomography-guided percutaneous access to the kidney can also be employed. It is slower and more cumbersome than nephrostomy placement under fluoroscopy but may be helpful in identifying a retrorenal colon and the location of the lung, pleura, liver, and spleen in upper pole access. Some of the indications for CT-guided percutaneous access include spinal dysraphism, morbid obesity, and abnormal anatomy [19–21]. The success of CT-guided percutaneous nephrostomy tube placement approaches 100% and may minimize the risk of major complications like bowel and visceral injury [22,23].
The use of US guidance during nephrostomy tube placement has some potential advantages compared to fluoroscopy and eliminates the need for ionizing radiation. US may result in shorter procedure times, fewer punctures, real-time visualization of surrounding structures, easier identification of posterior and anterior calyces and the ability to visualize and avoid the lung, pleura, and bowel [24]. Using ultrasound, successful nephrostomy placement has been reported in 91–100% of patients [25–27]. Major complications occurred in about 5% of patients [27].
The imaging used and the manner in which that imaging is employed may have significant effects upon the radiation exposure for patients during PCNL. Lipkin and colleagues used a validated phantom model to determine the effective dose during PCNL. The effective dose for left and right PCNL was 0.021 mSv/s and 0.014 mSv/s, respectively. These corrections were multiplied by the median fluoroscopy time of 386.3 and 545 sec for left and right PCNL, respectively. The effective dose received by the patient was 8.11 mSv on the left and 7.63 mSv on the right [28].
The fluoroscopic radiation exposure received by the patient is dependent upon patient factors, fluoroscopy settings, and the specifics of the machine. Newborns have a 3× higher risk of developing cancer compared to adults due to their longer life expectancy and greater susceptibility to the effects of ionizing radiation [29,30]. A patient of medium build may typically receive a skin entrance dose of 30 mGy/min [31]. Obesity increases radiation exposure due to poor radiation penetration and the x-ray source being closer to the patient [32]. An obese patient may receive a dose as high as 10–50× that of an individual with normal build [33,34].