

Second Edition
King’s College Hospital NHS Foundation Trust
UK
Maastricht Multimodal Molecular Imaging Institute
Mastricht University
The Netherlands
Department of Experimental and Clinical Toxicology
Saarland University
Germany
Formerly School of Biological and Chemical Sciences
Queen Mary University of London
UK
This edition first published 2020
© 2020 John Wiley & Sons, Ltd
Edition History
John Wiley & Sons, Ltd (1e, 2007)
All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, except as permitted by law. Advice on how to obtain permission to reuse material from this title is available at http://www.wiley.com/go/permissions.
The right of Robert J. Flanagan, Eva Cuypers, Hans H. Maurer and Robin Whelpton to be identified as the authors of this work has been asserted in accordance with law.
Registered Offices
John Wiley & Sons, Inc., 111 River Street, Hoboken, NJ 07030, USA
John Wiley & Sons Ltd, The Atrium, Southern Gate, Chichester, West Sussex, PO19 8SQ, UK
Editorial Office
The Atrium, Southern Gate, Chichester, West Sussex, PO19 8SQ, UK
For details of our global editorial offices, customer services, and more information about Wiley products visit us at www.wiley.com.
Wiley also publishes its books in a variety of electronic formats and by print-on-demand. Some content that appears in standard print versions of this book may not be available in other formats.
Limit of Liability/Disclaimer of Warranty
In view of ongoing research, equipment modifications, changes in governmental regulations, and the constant flow of information relating to the use of experimental reagents, equipment, and devices, the reader is urged to review and evaluate the information provided in the package insert or instructions for each chemical, piece of equipment, reagent, or device for, among other things, any changes in the instructions or indication of usage and for added warnings and precautions. While the publisher and authors have used their best efforts in preparing this work, they make no representations or warranties with respect to the accuracy or completeness of the contents of this work and specifically disclaim all warranties, including without limitation any implied warranties of merchantability or fitness for a particular purpose. No warranty may be created or extended by sales representatives, written sales materials or promotional statements for this work. The fact that an organization, website, or product is referred to in this work as a citation and/or potential source of further information does not mean that the publisher and authors endorse the information or services the organization, website, or product may provide or recommendations it may make. This work is sold with the understanding that the publisher is not engaged in rendering professional services. The advice and strategies contained herein may not be suitable for your situation. You should consult with a specialist where appropriate. Further, readers should be aware that websites listed in this work may have changed or disappeared between when this work was written and when it is read. Neither the publisher nor authors shall be liable for any loss of profit or any other commercial damages, including but not limited to special, incidental, consequential, or other damages.
Library of Congress Cataloging-in-Publication Data
Names: Flanagan, Robert J., author.
Title: Fundamentals of analytical toxicology : clinical and forensic / Robert J. Flanagan, King's College Hospital NHS Found Trust, UK, Eva Cuypers, Mastricht University, The Netherlands, Hans H. Maurer, Department of Experimental and Clinical Toxicology, Saarland University, Germany, Robin Whelpton, Formerly School of Biological and Chemical Sciences, Queen Mary University of London, UK.
Description: Second edition. | Hoboken, NJ : Wiley, 2020. | Includes bibliographical references and index.
Identifiers: LCCN 2020007512 (print) | LCCN 2020007513 (ebook) | ISBN 9781119122340 (cloth) | ISBN 9781119122364 (adobe pdf) | ISBN 9781119122371 (epub)
Subjects: LCSH: Analytical toxicology.
Classification: LCC RA1221 .F86 2020 (print) | LCC RA1221 (ebook) | DDC 615.9/07–dc23
LC record available at https://lccn.loc.gov/2020007512
LC ebook record available at https://lccn.loc.gov/2020007513
Cover Design: Wiley
Cover Images: © Xuanyu Han/Getty Images, © Alexander Limbach/Shutterstock
The analytical toxicologist may be required to detect, identify, and in many cases measure a wide variety of compounds in samples from almost any component of the body or in related materials such as residues in syringes or in soil. Many difficulties may be encountered. The analytes may include gases such as carbon monoxide, drugs, solvents, pesticides, metal salts, and naturally-occurring toxins. Some poisons may be individual chemicals and others complex mixtures. New drugs, pesticides, and other substances continually present novel challenges in analysis and in the interpretation of the results of the analysis. The analyte might be an endogenous compound such as acetone, or an exogenous compound such as a drug and/or metabolite(s) of the drug, whilst the sample matrix may range from urine to bone.
Many biological samples contain muscle, connective tissue, and so forth, which may have to be separated or degraded prior to an analysis, as well as a multitude of small and large molecular weight compounds. The concentration of the analyte to be measured can range from g L–1 (parts per thousand) in the case of blood ethanol to μg L–1 (parts per thousand million) in the case of plasma digoxin, and even ng L–1 (parts per million million) in the case of the potent opioid carfentanil. The stability of the analytes in biological samples also varies considerably, ranging from a few minutes for protease sensitive peptides and esters such as aspirin and diamorphine, to several years for some other drugs and pesticides.
This book aims to give principles and practical information on the analysis of drugs, poisons and other relevant analytes in biological and related specimens, particularly clinical and forensic specimens, i.e. it is a ‘toolkit’ in modern parlance. As such, this volume extends the scope of the World Health Organization (WHO) basic analytical toxicology manual1 and builds on the success of the first edition of this work that appeared in 2007.2 Moreover, it is intended to complement Dr Randall Baselt's Disposition of Toxic Drugs and Chemicals in Man (Edition 12. Seal Beach: Biomedical Publications, 2020), which remains the seminal reference work as regards the interpretation of analytical toxicology data.
A major difficulty in writing any textbook is deciding on the order of presentation. Having taken account not only of reviewer comments on the first edition, but also of the advances in analytical methods on the one hand, and the range of analyses that may now be required on the other, the material has been updated, expanded and presented in a new order. However, much of the discussion of the historical development of analytical toxicology present in the first edition has been removed to save space. On the other hand, some discussion of more traditional methods such as thin-layer chromatography has been retained for the simple reason that such methods are still used in many parts of the world.
After providing some background information, Section A outlines basic laboratory operations (aspects of sample collection, transport, storage, and disposal, use of internal standards, method implementation/validation, quality control and quality assessment, staff training, laboratory accreditation, etc.) and basic methodology ranging from simple colour tests through spectrophotometry to immunoassay and enzyme-based assays. Section B discusses separation science in detail (chromatography and electrophoresis, mass spectrometry, and ion mobility spectrometry). Section C reviews xenobiotic absorption, distribution and metabolism, and pharmacokinetics. Section D aims to unify this material and discusses point-of-contact testing, laboratory-based substance misuse and general toxicology screening, therapeutic drug monitoring, and trace elements and toxic metals analysis. The section concludes with a general discussion on the interpretation of analytical toxicology results.
This book is intended for use by scientists trained appropriately in laboratory work. Care should be taken to ensure the safe handling of all chemical and biological materials, and particular attention should be given to the possible occurrence of allergy, infection, fire, explosion, or poisoning (including transdermal absorption or inhalation of toxic vapours). Readers are expected to consult current local health and safety regulations and to adhere to them.
We have followed IUPAC nomenclature for chemical names except when Chemical Abstracts nomenclature or trivial names are more readily understood. With regard to symbols, we have adopted the convention that variables and constants are italicized, but labels and mathematical operators are not. Thus, for example, the acid dissociation constant is written Ka, K being the variable, a being a label to denote that it is an acid dissociation constant. The notation for the negative logarithm of Ka is pKa – p is a mathematical symbol and is not italicized. Where the subscript is a variable then it is italicized, so the concentration at time t, is Ct, but the concentration at time 0 is C0. Note especially that relative molecular mass (molecular weight, relative molar mass), the ratio of the mass of an atom or molecule to the unified atomic mass unit (u), is referred to throughout as Mr. The unified atomic mass unit, sometimes referred to as the dalton (Da), is defined as one twelfth of the mass of one atom of 12C. The symbol amu for atomic mass unit can sometimes be found, particularly in older works. The unified atomic mass unit is not a Système International (SI) unit of mass, although it is (only by that name, and only with the symbol u) accepted for use with SI.
As to drugs and pesticides, we have used recommended International Non-proprietary Name (rINN) or proposed International Non-proprietary Name (pINN) whenever possible. For misused drugs, the most common chemical names or abbreviations have been used. It is worth noting that for rINNs and chemical nomenclature, it is now general policy to use ‘f’ for ‘ph’ (e.g. sulfate not sulphate), ‘t’ for ‘th’ (e.g. chlortalidone not chlorthalidone) and ‘i’ for ‘y' (mesilate not mesylate for methanesulfonate, for example). However, so many subtle changes have been introduced that it is difficult to ensure compliance with all such changes. Names that may be encountered include the British Approved Name (BAN), the British Pharmacopoeia (BP) name, the United States Adopted Name (USAN), the United States National Formulary (USNF) name, and the United States Pharmacopoeia (USP) name. Where the rINN is markedly different from common US usage, for example acetaminophen rather than paracetamol, meperidine instead of pethidine, the alternative is given in parentheses at first use and in the index.
Isotopically-labelled compounds are indicated using the usual convention of square brackets to denote the substituted atoms, and site of substitution where known. For example, [2H3-N-methyl]-hyoscine indicates that the hydrogen atoms in the N-methyl group have been substituted by deuterium – this should not be confused with N-methylhyoscine (methscopolamine).
A useful source of information on drug and poison nomenclature is the Merck Index Online (www.rsc.org/Merck-Index/). Chemical Abstracts Service (CAS) Registry Numbers (RN) provide a unique identifier for individual compounds, but it is important to note that salts, hydrates, racemates, etc., each have their own RNs. Similarly, when discussing dosages we have tried to be clear when referring to salts, and when to free acids, bases, or quaternary ammonium compounds.
The oxidation number of metal ions is given by, for example, iron(II), but older terminology such as ferrous and ferric iron for Fe2+ and Fe3+, respectively, will be encountered in the literature.
We emphasize that cross-referral to an appropriate local or national formulary is mandatory before any patient treatment is initiated or altered. Proprietary names must be approached with caution – the same name is sometimes used for different products in different countries.
Uniform resource locators (URLs, web addresses) were correct at the time of printing. If the cited links are broken, readers should use an appropriate search engine or other resource to find the current URL unless directed otherwise.
In parts of Europe some laboratories report analytical toxicology data in ‘amount concentration’ using what have become known as SI molar units (μmol L–1, etc.), whilst others, especially in the US, continue to use mass concentration [so-called ‘traditional’ units (mg L–1, etc. or even mg dL–1)]. Most published analytical toxicology and pharmacokinetic data are presented in SI mass units per millilitre or per litre of the appropriate fluid [the preferred unit of volume is the litre (L)], or units that are numerically equivalent in the case of aqueous solutions:
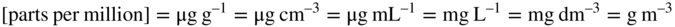
When preparing written statements for a court of law or other purpose outside the normal reporting channels it is advisable to write out the whole unit of measurement in full (milligrams per litre, for example).
An exception to the above is carboxyhaemoglobin saturation (COHb), which is usually reported as a percentage of the total haemoglobin present in the sample (% COHb) – the SI convention is that fractions of one should be used rather than percentages, but this is generally ignored.
We have followed the recommendations of the UK NPIS/ACB (National Poisons Information Service/Association for Clinical Biochemistry and Laboratory Medicine) for reporting analytical toxicology results and have used the litre as the unit of volume and SI mass units except for lithium (and sometimes toxic metals/trace elements), methotrexate, and thyroxine (NPIS/ACB. Laboratory analyses for poisoned patients: Joint position paper. Ann Clin Biochem 2002; 39: 328–39). More information on SI is available (Flanagan RJ. SI units – Common sense not dogma is needed. Br J Clin Pharmacol 1995; 39: 589–94).
Conversion from mass concentration (ρ) to amount concentration (c) (‘molar units’) and vice versa is simple if the molar mass (M) of the compound of interest is known. Thus, if a solution contains 302.4 g L–1 of a compound of Mr 151.2 g mol–1
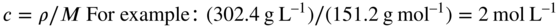
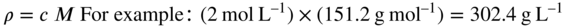
However, such conversions always carry a risk of error. Especial care is needed in choosing the correct Mr if the drug is supplied as a salt, hydrate, etc. This can cause great discrepancies especially if the contribution of the accompanying anion or cation is high. Most analytical measurements are reported in terms of the free acid or base, and not the salt.